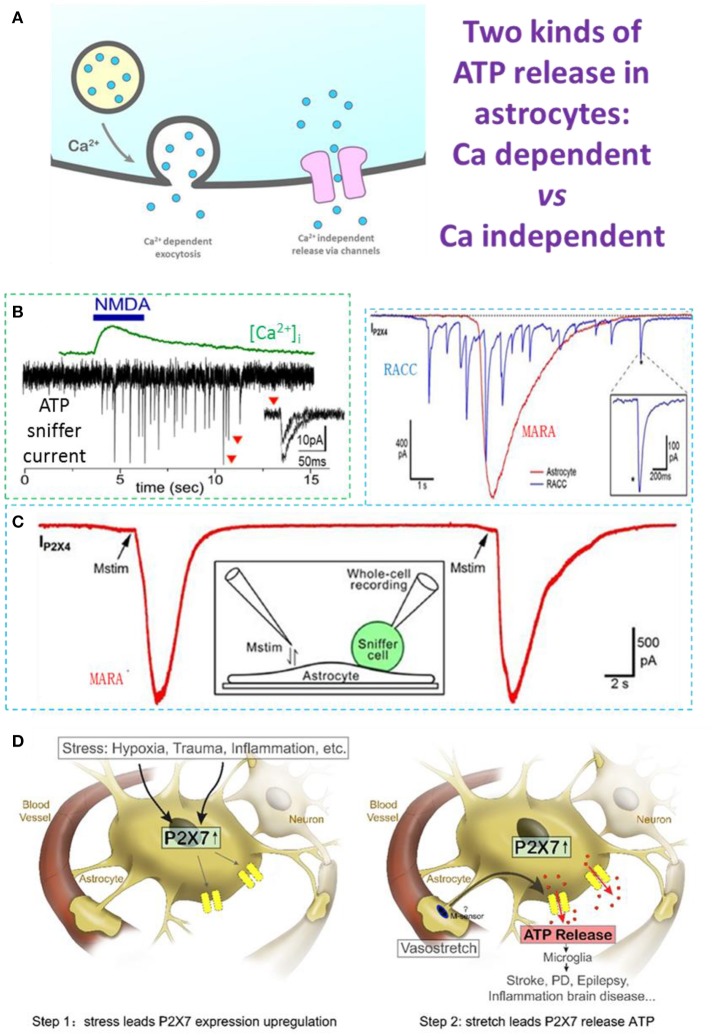
Figure 1.
Ca2+-dependent and Ca2+-independent ATP release in astrocytes. (A) Two types of ATP release: Ca2+-dependent quantal release and Ca2+-independent non-quantal release. In the Ca2+-dependent pathway, secretory vesicles with packaged ATP are trafficked to the plasma membrane where they dock and fuse with it on arrival of a stimulus; this typically depends on an increase of cytosolic Ca2+. In the Ca2+-independent pathway, ATP is released through channels expressed on the astrocyte plasma membrane, such as the swelling-induced anion channel, connexin hemichannels activated by lower Ca2+ concentrations, and ionotropic purinergic receptor channels. (B) N-methyl-D-aspartate (NMDA)-induced Ca2+-dependent quantal ATP release from a freshly-isolated astrocyte, recorded by a HEK293-P2X2 sniffer cell. (C) Typical MARA current (IP2X4) recorded with an HEK293A sniffer-cell expressing P2X4 (ATP sniffer) on an astrocyte. The astrocyte was stimulated twice and the MARA signal was reproducible. Inset, cartoon of the experimental protocol for MARA recording; Upper right: MARA vs Ca2+-dependent ATP release of burst quantal events from a rat chromaffin cell (RACC). (D) Two-step hypothesis of MARA-mediated brain diseases. Left. Step 1: Stress induces upregulation of P2X7 receptor expression in astrocytes. The stressors include hypoxia (i.e., stroke and ischemia), trauma, and CNS diseases associated with inflammation or neurodegeneration. Right. Step 2: Stretch leads to P2X7-mediated ATP release from astrocytes. The abundant ATP is an emergency signal for the neural immune system that recruits and activates microglia in the ATP “hot-spots” and promotes disease. All data adapted from Xiong et al. (2018), except B adapted from Lalo et al. (2014). All data with reproduction permission from the original publishers.