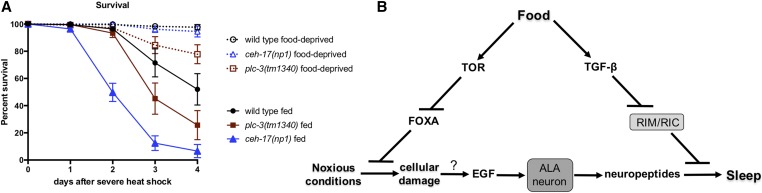
Figure 6.
FD reduces the need to sleep. (A) FD reduces the lethality associated with the SIS-defective ceh-17(np1) and plc-3(tm1340) mutations. Consistent with previous results (Hill et al. 2014), wild-type animals show a marked survival advantage over SIS-defective mutants (P < 0.0001, log-rank test) and here we find that food-deprived ceh-17 and plc-3 mutant animals show significantly greater survival than their fed counterparts (P < 0.0001, log-rank test). Percentage of animals surviving at daily intervals after a severe heat shock (40° for 20 min) are shown. Mean and ± SEM of a minimum of four trials, each containing at least 50 animals each, are shown. (B) Proposed model of starvation-induced plasticity in sleep drive. Under fed conditions, SIS requires DAF-7/TGF-β. In response to food scarcity, reduced DAF-7/TGF-β signaling allows activation of the RIM/RIC interneurons, potentially decreasing ALA target tissue competence to respond to sleep-promoting neuropeptides. Reduced nutrient availability and concomitant reduction of LET-363/TOR activity results in derepression of the PHA-4/FOXA transcription factor, leading to engagement of stress responses that potentially reduce the need to sleep. At this time the mechanism linking accrual of cellular damage to activation of EGF signaling in the ALA neuron is not known.