Abstract
Brown et al. report that two weeks of exogenous leptin administration to leptin-naive individuals with lipodystrophy resulted in increased energy expenditure and lipolysis, decreased ectopic liver fat, improved hepatic and peripheral insulin sensitivity, and attenuated dyslipidemia. Leptin withdrawal in individuals with lipodystrophy did not produce reciprocal effects on these phenotypes and resulted in significant improvements only in hepatic insulin sensitivity. This asymmetry in responses to leptin initiation and cessation is consistent with the other aspects of leptin biology that are dependent on the metabolic context in which this adipocyte-derived hormone functions.
Metabolic consequences of lipodystrophy
Individuals with lipodystrophy — either as a result of failure to develop adipocytes (congenital lipodystrophy) or loss of adipocytes (acquired lipodystrophy) — have variable degrees of hypoleptinemia relative to fat mass, depending on the type and severity of the disease (1). Affected individuals are hyperphagic, euthyroid, and eumetabolic in the case of congenital lipodystrophy (2), or hypermetabolic in the case of HIV-related lipodystrophy (3). Individuals with congenital lipodystrophy display increased sympathetic nervous system (SNS) and decreased parasympathetic nervous system (PNS) tone and deposit ectopic fat in muscle and liver. Despite their lack of adipose tissue, these individuals experience many of the comorbidities traditionally associated with obesity (4, 5), presumably by similar proximate cellular mechanisms.
Leptin therapy in individuals with lipodystrophy
The article by Brown et al. in this issue of the JCI describes a highly controlled, 19-day in-patient protocol examining the effects of initiation and cessation of leptin therapy on individuals with lipodystrophy (6). Briefly, subjects (80% with partial and 20% with generalized lipodystrophy) who had never received leptin therapy and others already receiving leptin (all of whom had generalized lipodystrophy) remained on their usual treatment while consuming a calculated weight maintenance diet for five days. For the next 14 days, while ingesting an isocaloric diet, leptin-naive subjects received metreleptin, and the drug was discontinued in subjects already receiving it.
Metreleptin initiation produced rapid improvements in insulin sensitivity (hepatic and peripheral, both of which were significantly correlated with changes in hepatic lipid content) and dyslipidemia (decreased total cholesterol and triglycerides), increased lipolysis, and decreased hepatic fat content and energy expenditure. In contrast, stopping metreleptin significantly decreased peripheral insulin sensitivity only, and changes in insulin sensitivity were not significantly correlated with hepatic lipid content.
Leptin in lipodystrophy versus other causes of hypoleptinemia
The ob gene product, now known as leptin, was originally pursued physiologically and then genetically as a regulatory molecule for body fat stores. Subsequent analyses in animal models and humans have indicated that the hormone’s primary physiological role is in signaling the sufficiency of somatic energy stores for survival and reproduction, i.e., in defending fat stores in the face of undernutrition rather than promoting weight loss in the case of overnutrition (7, 8). However, in the course of these studies, leptin has been shown to affect not only central nervous system (CNS) pathways defending energy stores, but also multiple central and peripheral endocrine, immunologic, and autonomic functions (9).
The effects of hypoleptinemia and of exogenous leptin administration are dependent on the metabolic milieu in which they are manifested. Hypoleptinemia following dietary weight loss is due primarily to a reduced lipid content per adipocyte rather than the reduced adipocyte numbers that occur in lipodystrophy (10). Both etiologies of hypoleptinemia are associated with increased hunger, and both are responsive to leptin repletion (1, 7). However, in contrast to the lipodystrophy-related hypoleptinemia phenotypes described above, weight-reduced individuals display reduced energy expenditure, thyroid axis suppression (euthyroid sick syndrome), and decreased SNS and increased PNS tone, all of which, with the exception of the increased PNS tone, are at least partially reversed by leptin repletion (7, 11).
Weight loss following bariatric surgery and low baseline circulating leptin concentrations (per unit of body fat) (12) represent additional distinct metabolic milieus of hypoleptinemia. Hypoleptinemia following bariatric surgery is not associated with the same degree of hyperphagia, hypometabolism, or responsiveness to leptin as nonsurgical dietary weight loss (13). Low circulating leptin concentrations at usual body weight constitute a significant risk factor for subsequent weight gain (14), but baseline leptin concentrations have also been reported to be negatively correlated with the amount of weight lost during out-patient caloric restriction (15). How obese (or nonobese) individuals with idiopathic low leptin per unit of fat mass might respond to low-dose exogenous leptin is an important question.
There are also context-sensitive differences in the associations of hypoleptinemic conditions with comorbidities. Dietary and surgical weight loss are associated with improvements in insulin sensitivity, hepatic steatosis, and dyslipidemia, all of which are characteristic comorbidities of lipodystrophy.
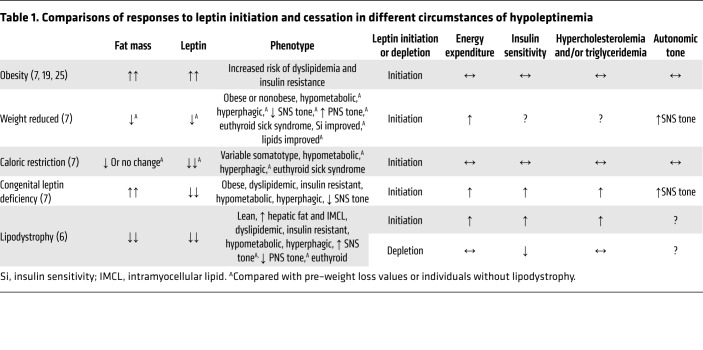
The different phenotypes associated with hypoleptinemia of different etiologies and changes in those phenotypes following initiation and cessation of leptin therapy (Table 1) clearly indicate that all hypoleptinemic states are not physiologically identical. The actions of leptin administration or withdrawal are affected by metabolic context — including the status of energy balance and energy stores — in which the hormone is administered or withdrawn.
Table 1. Comparisons of responses to leptin initiation and cessation in different circumstances of hypoleptinemia.
In both lipodystrophy and weight loss–induced hypoleptinemia, the effects of a decline and a repletion of leptin are not reciprocal. Leptin administration to subjects with lipodystrophy affects energy homeostasis, lipids, lipolysis, and hepatic triglycerides, which are not affected by withdrawal of exogenous leptin from patients with lipodystrophy (6). Leptin repletion in weight-reduced individuals increases serum T3 above the levels measured prior to weight loss, but does not reverse the increase in PNS tone that occurs as a result of weight loss (7).
Leptin physiology is metabolic context dependent
The bioactivity of leptin depends on both energy stores and energy balance at the time the hormone is administered (7). Hypoleptinemia following caloric restriction triggers hyperphagia, reduced energy expenditure, and neuroendocrine and autonomic changes that are largely reversed by leptin repletion (7), but there is little if any effect of leptin repletion during caloric restriction (an even more severe state of hypoleptinemia) or in euleptinemic lean or obese individuals on these same phenotypes (7, 16–19).
Perhaps the most striking differences between the effects of leptin repletion in subjects with lipodystrophy versus those with hypoleptinemia following weight loss are evident in their respective energy expenditures. In patients with lipodystrophy, cessation of leptin had no apparent effect on resting energy expenditure, while leptin repletion provoked a decline in resting and total energy expenditure. In similarly tightly controlled in-patient studies of experimental weight loss and subsequent leptin repletion, we have found that maintenance of reduced body weight is associated with significant declines in total, resting, and nonresting energy expenditure that are largely reversed by leptin repletion (7). In weight-reduced individuals fed an isocaloric diet, leptin increases energy expenditure and further reduces fat mass (7). Administration of leptin to hypoleptinemic lipodystrophic individuals fed an isocaloric diet decreased their apparent energy expenditure, while nonetheless being associated with loss of body weight (0.7 kg) and fat mass (0.2 kg) over the 2-week in-patient study period. The thermodynamics in this latter instance are incongruous, since a 0.2-kg loss of fat mass over 14 days would represent an approximately 140-kcal/day increase in energy expenditure relative to intake rather than the approximately 140-kcal/day decrease in 24-hour energy expenditure by chamber calorimetry reported in the leptin-initiation group (20).
Taken together, the lack of reciprocity of metabolic, anatomic, and energy homeostatic responses to leptin repletion and depletion in subjects with lipodystrophy (reduced adipocyte numbers) and the differential responses to leptin administration in subjects without lipodystrophy (reduced adipocyte volume) prior to, during, and after weight loss indicate that the physiological effects of leptin are context dependent and influenced by adipose tissue per se, energy balance, autonomic tone, and neuroendocrine ambients. The importance of adipose tissue per se as an important contributor to the metabolic environment that mediates the effects of leptin is illustrated by FAT-ATTAC ob/ob mice, in which induced adipocyte apoptosis causes more severe hyperphagia and impaired glucose-stimulated insulin secretion compared with responses attributable to leptin deficiency in the context of intact adipocytes (21).
Leptin initiation affects hepatic and peripheral insulin sensitivity, both of which correlate with hepatic lipid content. Leptin cessation only affects peripheral insulin sensitivity. These differences may indicate that the effects of leptin repletion and depletion on insulin sensitivity in lipodystrophy occur via different mechanisms, are dependent on the amount of hepatic triglyceride (roughly 4-fold higher at baseline in the leptin-initiation subjects), or that the apparent concordance of changes in hepatic and peripheral insulin sensitivity and hepatic triglycerides constitutes an association without a causal relationship.
The beneficial effects of leptin repletion on hepatic and peripheral insulin sensitivity, hepatic lipid content, and dyslipidemia in lipodystrophic and congenitally leptin-deficient states have been noted previously (22), though without the clear disassociation from changes in food intake that was achieved by Brown et al. (6). The abrupt cessation of leptin in the subjects with generalized lipodystrophy may be viewed as physiologically similar to the disproportionate (to fat mass), sharp declines in circulating leptin concentrations that occur during fasting, which is a metabolic state that is largely nonresponsive to leptin (23). The acute increase in circulating leptin that occurred in the initiation group with predominantly partial lipodystrophy may be comparable to leptin repletion following weight loss, which is a very leptin-sensitive state (7).
Concluding remarks
In summary, the study by Brown et al. demonstrates that, in individuals with lipodystrophy who had never been treated with leptin, repletion of leptin results in significant changes in glucose and lipid metabolism and energy expenditure. By design, these effects were independent of caloric intake but were neither identical to the effects of leptin repletion in the hypoleptinemic state following weight loss, nor reciprocal to the effects of leptin cessation in individuals with lipodystrophy. These findings, in the context of other studies of hypoleptinemic states, highlight the dependency of leptin functionality on metabolic ambience (24, 25). Understanding their biological bases is likely to lead to the identification of new adipokines.
Version 1. 07/16/2018
Electronic publication
Version 2. 08/01/2018
Print issue publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information: J Clin Invest. 2018;128(8):3237–3239. https://doi.org/10.1172/JCI122042.
Contributor Information
Michael Rosenbaum, Email: mr475@columbia.edu.
Rudolph L. Leibel, Email: rl232@columbia.edu.
References
- 1.Haque WA, Shimomura I, Matsuzawa Y, Garg A. Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab. 2002;87(5):2395. doi: 10.1210/jcem.87.5.8624. [DOI] [PubMed] [Google Scholar]
- 2.Taleban S, et al. Energy balance in congenital generalized lipdystrophy type I. Metabolism. 2008;57(8):1155–1161. doi: 10.1016/j.metabol.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vassimon H, de Paula F, Machado A, Monteiro J, Jordao A., Jr Hypermetabolism and altered substrate oxidation in HIV-infected patients with lipodystroph. Nutrition. 2012;28(9):912–916. doi: 10.1016/j.nut.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Faria C, et al. Autonomic modulatin in patients with congenital generalized lipodystrophy (Berardinelli-Seip syndrome) Europace. 2009;11(6):763–769. doi: 10.1093/europace/eup095. [DOI] [PubMed] [Google Scholar]
- 5.Diker-Cohen T, Cochran E, Gorden P, Brown RJ. Partial and generalized lipodystrophy: comparison of baseline characteristics and response to metreleptin. J Clin Endocrinol Metab. 2015;100(5):1802–1810. doi: 10.1210/jc.2014-4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown RJ, et al. Metreleptin-mediated improvements in insulin sensitivity are independent of food intake in humans with lipodystrophy. J Clin Invest. 2018;128(8):3504–3516. doi: 10.1172/JCI95476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenbaum M, Leibel RL. 20 years of leptin: role of leptin in energy homeostasis in humans. J Endocrinol. 2014;223(1):T83–T96. doi: 10.1530/JOE-14-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MG Myers, Leibel R, Seeley R, Schwartz M. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010;21(11):643–651. doi: 10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicholson T, Church C, Baker DJ, Jones SW. The role of adipokines in skeletal muscle inflammation and insulin sensitivity. J Inflamm (Lond) 2018;15:9. doi: 10.1186/s12950-018-0185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch J, Batchelor B. Adipose tissue cellularity in human obesity. Clin Endocrinol Metab. 1976;5(2):299–311. doi: 10.1016/S0300-595X(76)80023-0. [DOI] [PubMed] [Google Scholar]
- 11. Leibel RL, Rosenbaum M. Metabolic responses to weight perturbation. In: Clément K, Spiegelman BM, Christen Y, eds. Novel Insights into adipose cell functions, research and perspectives in endocrine interactions. Heidelberg, Germany: Springer-Verlag; 2010:121–133. [Google Scholar]
- 12.Kilpeläinen TO, et al. Genome-wide meta-analysis uncovers novel loci influencing circulating leptin levels. Nat Commun. 2016;7:10494. doi: 10.1038/ncomms10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korner J, et al. Randomized double-blind placebo-controlled study of leptin administration after gastric bypass. Obesity (Silver Spring) 2013;21(5):951–956. doi: 10.1002/oby.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravussin E, Gautier J. Metabolic predictors of weight gain. Int J Obes. 1999;23(suppl 1):S37–S41. doi: 10.1038/sj.ijo.0800793. [DOI] [PubMed] [Google Scholar]
- 15.Labayen I, Ortega FB, Ruiz JR, Lasa A, Simón E, Margareto J. Role of baseline leptin and ghrelin levels on body weight and fat mass changes after an energy-restricted diet intervention in obese women: effects on energy metabolism. J Clin Endocrinol Metab. 2011;96(6):E996–1000. doi: 10.1210/jc.2010-3006. [DOI] [PubMed] [Google Scholar]
- 16.Rosenbaum M, Nicolson M, Hirsch J, Murphy E, Chu F, Leibel RL. Effects of weight change on plasma leptin concentrations and energy expenditure. J Clin Endocrinol Metab. 1997;82(11):3647–3654. doi: 10.1210/jcem.82.11.4390. [DOI] [PubMed] [Google Scholar]
- 17.Hukshorn CJ, van Dielen FM, Buurman WA, Westerterp-Plantenga MS, Campfield LA, Saris WH. The effect of pegylated recombinant human leptin (PEG-OB) on weight loss and inflammatory status in obese subjects. Int J Obes Relat Metab Disord. 2002;26(4):504–509. doi: 10.1038/sj.ijo.0801952. [DOI] [PubMed] [Google Scholar]
- 18.Ranganathan S, Ciaraldi TP, Henry RR, Mudaliar S, Kern PA. Lack of effect of leptin on glucose transport, lipoprotein lipase, and insulin action in adipose and muscle cells. Endocrinology. 1998;139(5):2509–2513. doi: 10.1210/endo.139.5.5980. [DOI] [PubMed] [Google Scholar]
- 19.Moon HS, et al. Leptin’s role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr Rev. 2013;34(3):377–412. doi: 10.1210/er.2012-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roffey DM, Byrne NM, Hills AP. Day-to-day variance in measurement of resting metabolic rate using ventilated-hood and mouthpiece & nose-clip indirect calorimetry systems. JPEN J Parenter Enteral Nutr. 2006;30(5):426–432. doi: 10.1177/0148607106030005426. [DOI] [PubMed] [Google Scholar]
- 21.Pajvani U, et al. Fat apoptosis through targeted activation of caspase8: a new mouse modible of inducible and reversible lipoatrophy. Nat Med. 2005;11(7):797–803. doi: 10.1038/nm1262. [DOI] [PubMed] [Google Scholar]
- 22.Tchang BG, Shukla AP, Aronne LJ. Metreleptin and generalized lipodystrophy and evolving therapeutic perspectives. Expert Opin Biol Ther. 2015;15(7):1061–1075. doi: 10.1517/14712598.2015.1052789. [DOI] [PubMed] [Google Scholar]
- 23.Perry RJ, Shulman GI. The role of leptin in maintaining plasma glucose during starvation. Postdoc J. 2018;6(3):3–19. doi: 10.14304/surya.jpr.v6n3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flier J, Maratos-Flier E. Leptin’s physiological role: Does the emperor of energy balance have no clothres? Cell Metab. 2017;26(1):24–26. doi: 10.1016/j.cmet.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Chan JL, Mietus JE, Raciti PM, Goldberger AL, Mantzoros CS. Short-term fasting-induced autonomic activation and changes in catecholamine levels are not mediated by changes in leptin levels in healthy humans. Clin Endocrinol (Oxf) 2007;66(1):49–57. doi: 10.1111/j.1365-2265.2006.02684.x. [DOI] [PubMed] [Google Scholar]