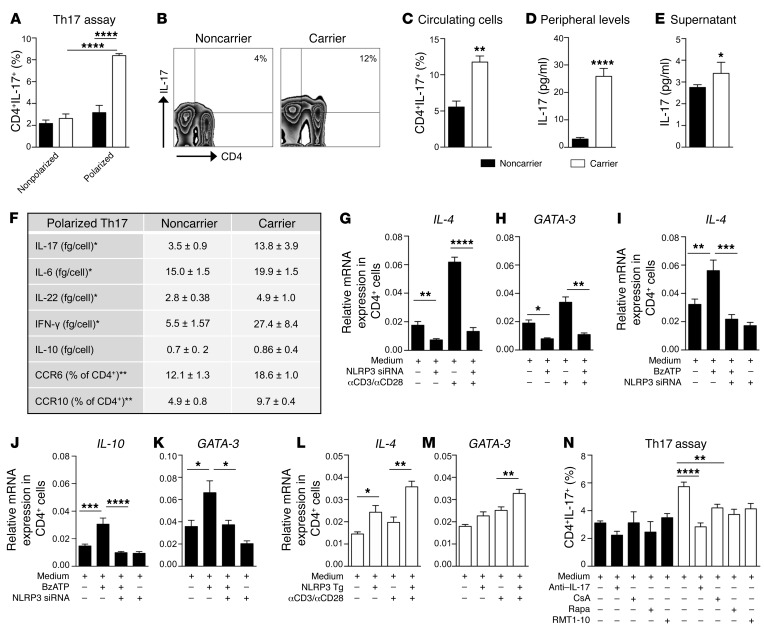
Figure 3. The dysregulated P2X7R/NLRP3 pathway is associated with an abnormal human Th cell immunophenotype.
(A) Percentage of in vitro–generated Th17 cells obtained from CD4+ T cells of carrier and noncarrier patients (n = 8). (B and C) Representative flow zebra plots (B) and quantitative histogram (C) depicting the percentage of peripheral CD4+IL-17+ cells (n = 8). (D) IL-17 plasma levels of carrier and noncarrier patients (n = 10). (E) IL-17 levels (Luminex) measured in the supernatants of unstimulated 24-hour-cultured CD4+ T cells of carrier and noncarrier patients (n = 5). (F) Table summarizing the secretome profile (Luminex, n = 5) and primary phenotypic characteristics (flow cytometry, n = 4) of carrier and noncarrier polarized Th17 cells. (G and H) Normalized mRNA expression of Th2-related factors IL-4 (G) and GATA-3 (H) measured in noncarrier CD4+ T cells exposed to transient knockdown of NLRP3 using silencing RNA (siRNA), before and after anti-CD3-Ig/anti-CD28-Ig stimulation (n = 3). (I–K) Normalized mRNA expression of the Th2-related factors IL-4 (I), IL-10 (J), and GATA-3 (K) measured in noncarrier CD4+ T cells exposed to transient knockdown of NLRP3 (siRNA), upon BzATP exposure (n = 4). (L and M) Normalized mRNA expression of the Th2-related factors IL-4 (L) and GATA-3 (M) measured in carrier CD4+ T cells, in which NLRP3 was overexpressed, before and after anti-CD3-Ig/anti-CD28-Ig stimulation (n = 3). (N) Effects of various treatments (anti–IL-17 antibody, RMT1-10, cyclosporin A [CsA] and rapamycin [Rapa]) on in vitro–generated Th17 cells (n = 5). Experiments were run in triplicate (D, G, H, and N) or in duplicate (F and I–M). mRNA expression was normalized to ACTB. Bars represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; Student’s t test or 1-way ANOVA with Bonferroni’s post hoc test.