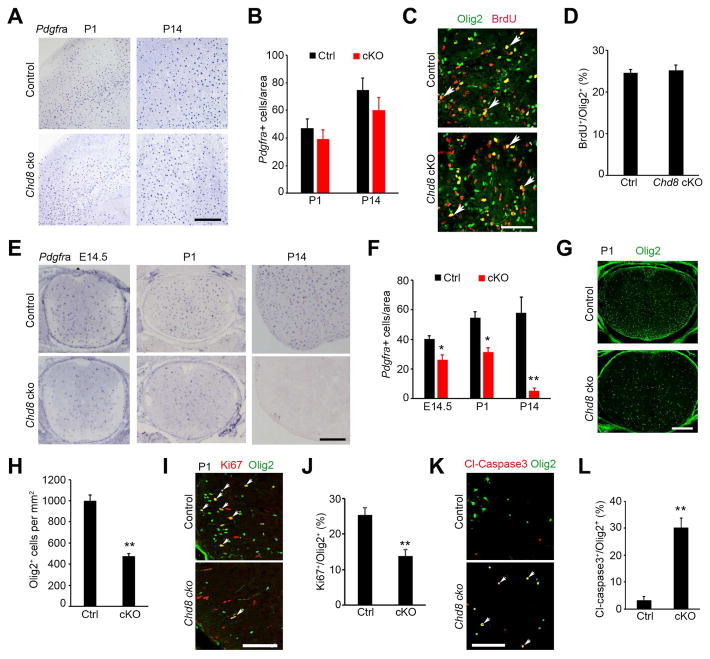
Figure 3. CHD8 is required for OPC generation and survival in the spinal cord but not in the cortex.
(A) In situ hybridization for Pdgfra in the cortices at P1 and P14. Scale bar, 300 μm.
(B) Quantification of Pdgfra+ OPCs in the cortex (n = 3 animals).
(C) Immunostaining for BrdU and Olig2 in the corpus callosum from P1 mice. Scale bar, 50 μm.
(D) Percentage of BrdU+ cells relative to all Olig2+ OPCs in the corpus callosum of P1 mice. (n = 3 animals).
(E,F) In situ hybridization for Pdgfra in the spinal cord of control and Chd8 cKO mice at E14.5, P1, P14. Scale bar, 300 μm. Panel F, quantification of Pdgfra+ OPCs.
(G,H) Olig2 immunostaining in the spinal cord of control and Chd8 cKO mice at P1. Scale bar, 200 μm. Panel H, quantification of Olig2+ cells.
(I) Olig2 and Ki67 immunostaining in the spinal cord of control and Chd8 cKO mice at P1. Scale bar, 100 μm.
(J) Quantification of Ki67+ relative to total Olig2+ OPCs.
(K) Primary OPCs from control and Chd8 cKO mice cultured in Sato medium without PDGFAA and NT3 for 24 hr were immune-stained with cleaved caspase 3 and Olig2. Scale bar, 100 μm.
(L) Percentage of cl-caspase3+ among total Olig2+ cells from above control and Chd8 cKO OPCs.
n = 3 animals/genotype; * p < 0.05 and ** p < 0.05, two-tailed unpaired Student’s t test in F, H, J, L.
See also Figure S3.