Fig. 1. One-step preparation of the poly(TA-DIB-Fe) copolymer network and its characterization.
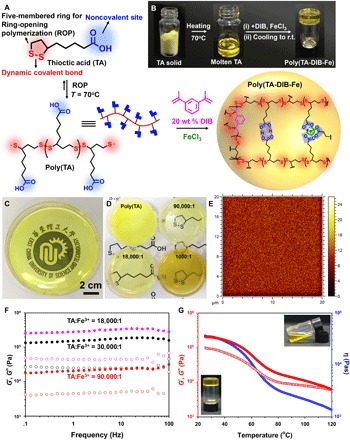
(A) Schematic representation of the synthesis route of the copolymer network. (B) Photographs of TA powder, molten TA liquid, and poly(TA-DIB-Fe) copolymer solid. r.t., room temperature. (C) The transparent polymeric film prepared by 25-g poly(TA-DIB-Fe) cured in a Petri dish with a TA-to-iron(III) molar ratio of 18,000:1. (D) Photographs of poly(TA) and poly(TA-DIB-Fe) film samples synthesized by different TA-to-iron(III) molar ratios. (E) Time-of-flight secondary ion mass spectroscopy (TOF-SIMS) mapping images of molecular ion fragments attributed to TA [TA-to-iron(III) molar ratio of 18,000:1]. (F) Frequency dependency of storage (solid dots, G′) and loss (hollow dots, G″) moduli of copolymer network with different iron(III) concentrations. (G) Temperature dependency of storage (solid dots, G′) and loss (hollow dots, G″) moduli and viscosity (blue dots, η) of copolymer network [TA-to-iron(III) molar ratio of 18,000:1]. Inset photographs manifest the transformation from solid to liquid due to the decrease in viscosity by heating.
