Abstract
Purpose
Many people travel abroad to access fertility treatments. This growing phenomenon is known as cross border reproductive care (CBRC) or fertility tourism. Due to its complex nature and implications worldwide, CBRC has become an emerging dilemma deserving more attention on the global healthcare agenda.
Methods
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, a systematic review of the literature was performed for all relevant full-text articles published in PubMed in English during the past 18 years to explore CBRC phenomenon in the new millennium.
Results
Little is known about the accurate magnitude and scope of CBRC around the globe. In this systematic and critical review, we identify three major dimensions of CBRC: legal, economic, and ethical. We analyze each of these dimensions from clinical and practical perspectives.
Conclusion
CBRC is a growing reality worldwide with potential benefits and risks. Therefore, it is very crucial to regulate the global market of CBRC on legal, economic, and ethical bases in order to increase harmonization and reduce any forms of exploitation. Establishment of accurate international statistics and a global registry will help diminish the current information gap surrounding the CBRC phenomenon.
Keywords: Cross border reproductive care, Fertility tourism, Sperm donation, Oocyte donation, Embryo donation, Surrogacy, Fertility preservation
Introduction
As the practice and delivery of fertility treatments have become more global, many people are traveling internationally to obtain fertility treatments. This growing phenomenon defined as cross border reproductive care (CBRC) is also known as fertility tourism, reproductive tourism, procreative tourism, transnational reproduction, reproductive travel (“reprotravel”), or reproductive exile. The most common forms of fertility treatments provided within CBRC are in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI), sperm donation, egg donation, embryo donation, commercial surrogacy, pre-implantation genetic diagnosis (PGD), sex selection, and fertility preservation. Various groups may seek fertility treatments in CBRC, such as infertile couples, single, gay, or transgender people and even patients of advanced age. The main reasons for CBRC are legal restrictions and high costs in home countries, as well as quality of care concerns (e.g., success rates, long waits, iatrogenesis), and sociocultural considerations (e.g., desires for privacy and linguistic and cultural familiarity) [1–10].
Due to its complex nature and implications worldwide, CBRC has become an emerging dilemma on the global healthcare agenda and yet little is known about its accurate magnitude and scope. In this systematic and critical review, we explore CBRC in the new millennium as a growing global phenomenon with multidimensional implications.
Methods
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11], a systematic review of the literature was performed for all relevant full-text articles published in PubMed in English throughout the past 18 years to explore the CBRC phenomenon in the new millennium. Based on these inclusion criteria, the following electronic search strategy was performed in PubMed: (cross border reproductive care AND full text[sb] AND (“2000/01/01”[PDat]: “2018/01/01”[PDat]) AND English[lang]). The full-text articles identified from the initial search underwent screening for titles and abstracts and were then checked for eligibility according to the inclusion criteria. Only the full-text articles that focus completely or partially on CBRC were included and fully reviewed. Data were extracted from the text, tables, graphs, and references of the included articles.
Results
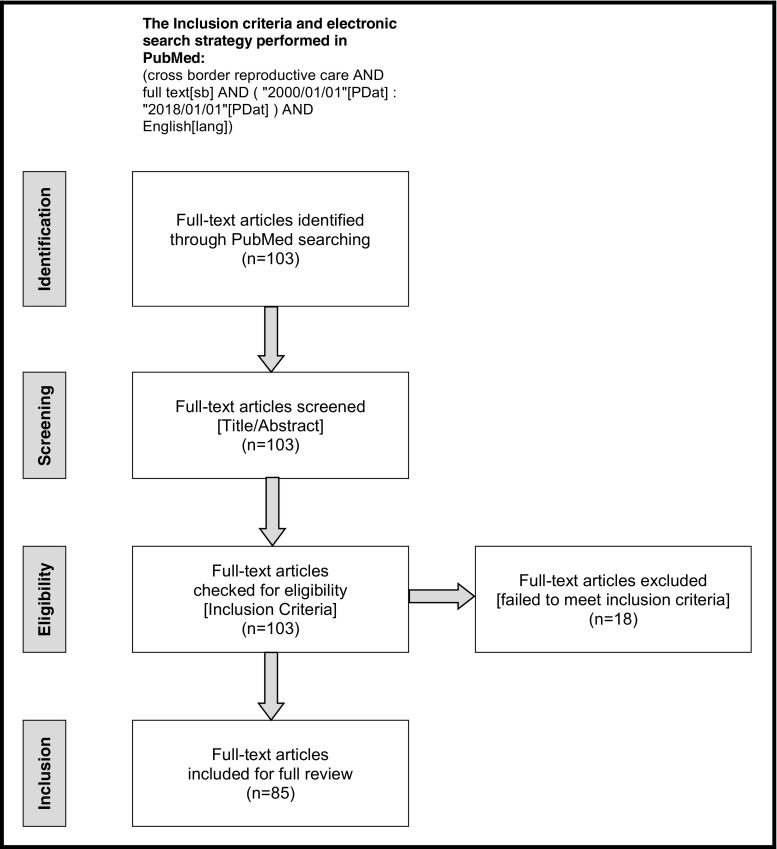
A total of 103 full-text articles were identified from the initial search; ~ 54% of them were published in the past 5 years, confirming the growing attention toward the CBRC topic. After screening titles and abstracts, all 103 full-text articles were checked for eligibility according to the inclusion criteria. Only 18 full-text articles were excluded, while the remaining 85 full-text articles that focused completely or partially on CBRC were included and fully reviewed. PRISMA flow diagram of the systematic review process is illustrated in Fig. 1. Some significant articles were not identified from the initial search but these were reviewed along with several recent books on the CBRC topic. Therefore, the final reference list was generated on the basis of originality and relevance to the broad scope of this review.
Fig. 1.
PRISMA four-phase flow diagram of identification, screening, eligibility, and inclusion steps
Although CBRC is a subtype of cross-border healthcare and medical tourism, little is known about its actual magnitude and scope around the globe. In this systematic and critical review, we identified three major issues of concern in CBRC: legal, economic, and ethical. Here, we analyze each of these dimensions from clinical and practice perspectives.
Discussion
Legal dimension of CBRC
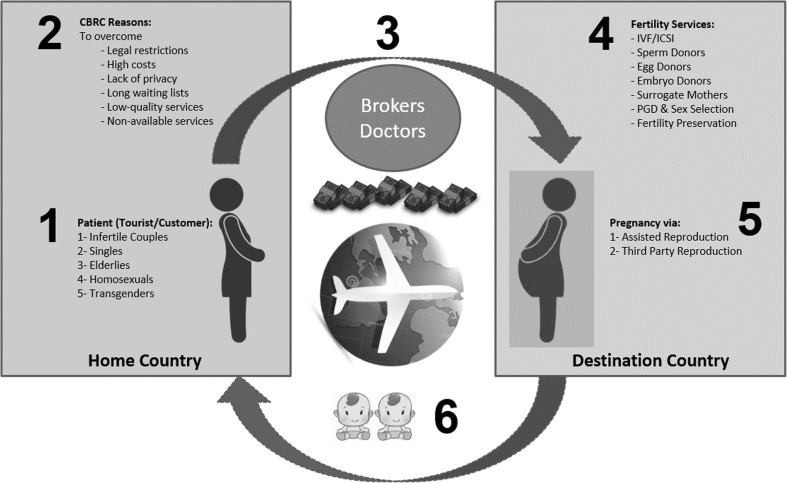
CBRC is a complex global phenomenon as we illustrate in Fig. 2. Undoubtedly, legal restriction in the home country is one of the most important reasons for CBRC. Usually, people travel abroad to less legally restrictive countries in order to receive fertility treatments that are restricted in their home countries [12–22]. Examples of more and less legally restrictive countries are listed in Tables 1 and 2 [23–25].
Fig. 2.
Cross border reproductive care (CBRC) cycle as a complex global phenomenon. (1) Different groups seeking CBRC. (2) Reasons of CBRC. (3) Intermediaries of CBRC including paid brokers and doctors abroad. (4) Fertility services provided as CBRC in the destination country. (5) Pregnancies achieved in CBRC via assisted or third party reproduction. (6) Take-home baby outcome of CBRC with increased incidence of twins
Table 1.
Examples of more and less legally restrictive countries in cross border reproductive care
| Countries | Specific legislation for assisted reproduction | IVF/ICSI | Gamete donation (sperm, egg, embryo) | Surrogacy | PGD and sex selection | Fertility preservation | |
|---|---|---|---|---|---|---|---|
| More legally restrictive countries | Germany | Yes | Allowed only for heterosexual couples | Sperm and embryo donations are allowed under certain conditions | Forbidden | Allowed only for medical reasons | Allowed |
| Austria | Yes | Allowed only for heterosexual couples | Sperm donation is allowed under certain conditions. Egg donation is recently allowed | Forbidden | Allowed only for medical reasons | Allowed | |
| Italy | Yes | Allowed only for heterosexual couples | Sperm and egg donation are recently allowed | Forbidden | Allowed only for medical reasons | Allowed | |
| UK | Yes | Allowed only for heterosexual couples and single women | Allowed | Altruistic is allowed | Allowed only for medical reasons | Allowed | |
| Egypt | No | Allowed only for heterosexual married couples | Forbidden | Forbidden | Allowed | Allowed | |
| Saudi Arabia | No | Allowed only for heterosexual married couples | Forbidden | Forbidden | Allowed | Allowed | |
| Less legally restrictive countries | Spain | Yes | Allowed for everyone | Allowed | Forbidden | Allowed | Allowed |
| Belgium | Yes | Allowed for everyone | Allowed | Forbidden | Allowed | Allowed | |
| Czech Republic | Yes | Allowed for everyone | Allowed | Forbidden | Allowed | Allowed | |
| USA | Yes | Allowed for everyone | Allowed | Allowed in some states | Allowed | Allowed | |
| India | Yes | Allowed for everyone | Allowed | Commercial is recently forbidden | Allowed | Allowed | |
| Israel | Yes | Allowed for everyone | Allowed | Altruistic is allowed | Allowed | Allowed | |
| Russia | Yes | Allowed for everyone | Allowed | Allowed | Allowed | Allowed | |
| Ukraine | Yes | Allowed for everyone | Allowed | Allowed | Allowed | Allowed | |
Table 2.
Examples of more and less legally restrictive countries regarding surrogacy
| Countries | Surrogacy laws by country (examples) |
|---|---|
| More legally restrictive countries | Countries where all kinds of surrogacy are forbidden by law: Germany, France, Belgium, Spain, Italy, Switzerland, Austria, Norway, Sweden, Iceland, Estonia, Moldova, Turkey, Saudi Arabia, Egypt, Other Arab Countries, Pakistan, China, Japan, Canada (Quebec), USA (Arizona, Michigan, Indiana, North Dakota). Countries where only altruistic surrogacy is allowed by law: India, Australia, Canada (except Quebec), United Kingdom, Netherlands, Denmark, Hungary, Israel, United States (New York, New Jersey, New Mexico, Nebraska, Virginia, Oregon, Washington) |
| Less legally restrictive countries | Countries where both commercial and altruistic surrogacy are allowed by law: Russian Federation, Ukraine, Belarus, Georgia, Armenia, Cyprus, South Africa, USA (Arkansas, California, Florida, Illinois, Texas, Massachusetts, Vermont) |
In vitro fertilization and intracytoplasmic sperm injection
IVF and ICSI are legally allowed in almost all countries worldwide. However, most countries vary in their legal restrictions regarding the availability of these treatments to older women, unmarried couples, single individuals, gay and transgender patients. As a result, thousands of infertile patients go abroad for IVF and ICSI [26–36]. Due to higher success rates and fewer regulations, some countries are known to be destinations for IVF and ICSI, such as the USA, Spain, Czech Republic, Denmark, Belgium, and Israel. In Europe, some countries have restrictions on the number of embryos to transfer. For that reason, many women from Germany, Italy, and the UK travel abroad to seek unrestricted IVF/ICSI treatments. The most common European destinations for such women are Spain, Czech Republic, and Belgium [37–44].
Gamete (sperm, egg, and embryo) donation
Third party reproduction, such as sperm, egg, and embryo donation, is not legally allowed in many countries due to ethical and religious reasons. Because of that, gamete donation is one of the most common reasons for CBRC on the global level.
Sperm donation is not allowed in many countries and is not widely available in some countries that do allow it. The UK is one example of a country that suffers from a shortage of sperm donors and has months-long waiting lists for sperm donation since donor anonymity was removed. Consequently, many UK patients import sperm or travel abroad to Denmark, Spain, and Belgium to obtain donor sperm [45, 46]. Denmark is a very well-known market for sperm donation due to its liberal legislation and the appeal of its donor population to those needing donor sperm, particularly in Europe. Denmark also allows the use of both anonymous and directed sperm donation for lesbian and single women. Many sperm banks in Denmark are aware of this type of business and have established advanced systems for online searching and for exporting donated sperm [47].
Egg or oocyte donation is considered illegal in many countries worldwide, including European countries such as Germany and Switzerland. Therefore, many infertile couples from these countries travel to other countries where egg donation is allowed. In Europe, Spain and Czech Republic are the most common destinations for egg donation [48, 49]. Almost half of all egg donation procedures in Europe are performed in Spain. Most fertility clinics in Spain and Czech Republic offer online searching of egg donor registries; donors must be under 35 years old, anonymous, and compensated for their time [50].
Embryo donation is not allowed in many countries worldwide; however, countries allowing it vary in the regulations allowing this treatment for unmarried couples, as well as single, gay, and transgender individuals. The primary countries allowing embryo donation are Spain, Czech Republic, Belgium, USA, and Russia [23–25, 51].
Surrogacy
Some women cannot carry a pregnancy due to congenital anomalies or some other disease that interferes with normal pregnancy. Also, some female cancer survivors cannot get pregnant due to side effects of gonadotoxic chemotherapy and radiotherapy. If those women want children, they may then consider altruistic or commercial surrogacy. Surrogacy is not allowed by law in many countries worldwide and in some American states due to ethical and religious reasons [52]. Examples of more- and less-legally restrictive countries regarding altruistic and commercial surrogacy are listed in Table 2. Worldwide, India had been the main destination for commercial surrogacy when it was still allowed by law and offered at low costs with satisfactory results. The cost of commercial surrogacy in India averaged about USD 20,000, but some Indian centers provided the service even at lower prices (USD 10,000). Usually the cost included compensation for the surrogate mother, in vitro fertilization, prenatal care, and delivery of the baby. In comparison to the cost of surrogacy in other countries—for example, the USA, where the total cost of surrogacy could reach USD 100,000 per case, India was considerably less expensive [53–55]. However, in 2017, India changed its surrogacy procedures, disallowing it for both non-Indian citizens and non-residential Indians (NRIs) in the diaspora. As a result, Russia is now one of the most well-known destinations for surrogacy tourism worldwide. Many European patients prefer to travel to Russia (rather than to countries such as India in the global South) due to a common European culture and better healthcare standards. Russia is also known for its liberal laws regarding fertility treatments. Fertility tourists have the same rights as Russian citizens for all assisted reproductive techniques. In Russia, the tourist couple using a surrogate can obtain a birth certificate for their baby regardless of the biological relation to the child [56].
Pre-implantation genetic diagnosis and sex selection
Another reason for CBRC is pre-implantation genetic diagnosis and sex selection. PGD (now called PGT-M; pre-implantation genetic testing for monogenic/single gene diseases) and sex selection are not allowed in many European countries, including the UK and Germany, except for screening of genetic and inherited disorders. Worldwide, the major destination for unrestricted PGD and sex selection is the USA [57].
Fertility preservation
As a newer technology, fertility preservation for medical or elective reasons is not widely available in many countries worldwide. The most common forms of fertility preservation are cryopreservation of sperm, eggs, embryos, as well as ovarian and testicular tissue. Advances in cryopreservation techniques have enabled successful international shipment of frozen gametes from one country to another, increasing the potential of a global CBRC market to develop in the new millennium [58–60]. Recently, elective (social) egg freezing or oocyte cryopreservation for non-medical reasons has emerged in clinical practice as a way for single women to preserve their fertility and achieve motherhood in the future [61, 62]. Some countries, such as China and Singapore, have outlawed elective fertility preservation. However, fertility preservation techniques usually do not face legal restrictions or ethical debates in terms of CBRC, except when cryopreserved sperm, eggs, and embryos are donated [63, 64]. The most common destinations for fertility preservation are Belgium, Denmark, Germany, USA, and Israel.
Economic dimension of CBRC
There are very few economic studies relating specifically to CBRC. Most studies are general economic evaluations of different assisted reproductive techniques (ART), rather than particular studies of CBRC [65–69]. The main reasons for lack of economic studies about CBRC are scarcity of information and absence of national and global registries [70].
In general, many economists consider fertility treatment as a low medical priority compared to other types of medical treatments. Therefore, national health insurance plans in many countries, even in Europe, do not fund fertility treatments well. As a result, most fertility treatments are either inadequately reimbursed or not reimbursed at all, and are usually out-of-pocket services. One of the fundamental concepts of economic theory is that individuals usually act in a rational way to maximize their welfare at the lowest cost possible. When infertile patients cannot get reimbursed fertility treatments in their home country, they try to find the most economical treatment anywhere else. In many cases, the desire of infertile patients to have children is great enough to push them to solve their problem through CBRC, even with limited financial capabilities. On the other hand, many fertility services are available with good success rates and attractive prices in many countries worldwide. Given the demand of infertile patients to have children and the supply of fertility services in many countries, the global CBRC industry has gradually developed over time [1–3, 65–70].
The major global markets or hubs for the CBRC industry are as follows: (1) Belgium and Israel for IVF, (2) Denmark for sperm donation, (3) Spain and Czech Republic for egg and embryo donation, (4) India, Russia, and the USA for commercial surrogacy (although India has now removed itself from this market), (5) the USA for PGD and sex selection, and (6) Denmark, Belgium, Germany, and the USA for fertility preservation.
From an economic perspective, CBRC carries some risks including the following: unclear information, supplier-induced demand, increased multiple pregnancy rates with increased costs incurred later in the home country, and shifting of scarce medical resources from the public to private sector in the destination country [1–3, 65–70].
The problem of unclear information is mainly due to receiving limited information about the prices of the fertility services abroad. Fertility treatments are complex and in most cases the detailed information about the different treatment options and their related costs are not adequately provided by the CBRC intermediaries (the “brokers”) and the suppliers (the “clinics”). Usually, the main source of information is the internet, but some language barriers may exist if the infertile patients do not speak the language of the destination country. In addition, CBRC brokers can lack transparency with little information available about them, their services, and remuneration. The problem of incomplete information leads consequently to other problems, including inability of infertile patients to make the best decision regarding their treatment and the best possible destination.
Supplier-induced demand refers to the situation where a healthcare provider makes treatment decisions based on economic reasons more than medical ones. In the CBRC context, limited information, out-of-pocket payments, time constraints, desire to increase the success rates of the infertility treatments, competition between fertility centers, and weak or absent legal regulations can lead to supplier-induced demand. Obviously, supplier-induced demand pushes doctors to use more expensive or aggressive treatment protocols to increase the success rates. Consequently, this may lead to increased risks of maternal and fetal complications, as well as increased multiple pregnancies rates and then increased costs incurred later in the home country [1–3, 65–70].
Multiple pregnancies come as a result of transferring more than one embryo during the ART cycle. Unfortunately, this is common in CBRC due to the great emotional desire of infertile patients to become pregnant, as well as the great desire of physicians to increase their success rates and satisfy their patients. From a clinical perspective, multiple pregnancy carries more maternal, fetal, and neonatal risks than a singleton pregnancy. Increased multiple pregnancy rates may subsequently increase the costs incurred later in the home country. This particular phenomenon was observed in the past in the UK [71].
A successful CBRC in the destination country may carry the risk of shifting scarce medical resources from the public to the private sector. However, the destination country can benefit from a successful CBRC by creation of new jobs.
From an economic perspective, most CBRC risks can be reduced by sharing adequate information between suppliers (the “clinics”), intermediaries (the “brokers”), consumers (the “infertile patients”), and all other stakeholders involved in this industry. Establishment of accurate international statistics and a future global registry will help diminish the current information gap.
Ethical dimension of CBRC
Unquestionably, CBRC raises many ethical, social, and religious debates. Some of these debates are old and related to assisted reproduction techniques (ART) in general, and other debates are new and related directly to CBRC itself [72–76]. For example, some infertile couples must become “law evaders,” traveling surreptitiously to other countries to evade the restrictive laws in their home countries. To overcome the problem of law evasion, cross border legal harmonization may be required, so that patients (and gametes) do not have to move from “restrictive” to “permissive” countries [77].
As an industry, CBRC involves many different parties, including patients, doctors, brokers, donors, and surrogates (Fig. 2). Each of those parties has its own goals and corresponding ethical and social dilemmas as well. In general, CBRC often involves relatively wealthy patients, the “fertility tourists,” and relatively poor donors and surrogates through a process facilitated by brokers with treatments carried out by doctors in clinics abroad [78–84].
Patients
Whether rich or poor, infertile patients often have strong desires to have children by any means possible. In many Western and developed societies, women are increasingly delaying childbearing in order to complete their education, improve their career, or find the right partner. Consequently, many Western women reach age 35 without having any children, and advanced maternal age is a common factor in infertility. In other words, delayed childbearing in many Western and developed countries is a very important underlying factor in the CBRC phenomenon [85–88].
In addition, other groups seeking CBRC include unmarried couples, single, gay, transgender, and older individuals. The heterogeneity of potential CBRC patients usually opens critical debates on whether a society is “liberal” in its attitudes toward LGBTQI (Lesbian, Gay, Bisexual, Transgender, Queer, Intersex) and other groups’ civil rights [89]. The Ethics Committee of the American Society for Reproductive Medicine (ASRM) encourages programs to “treat all requests for assisted reproduction equally without regard to marital status or sexual orientation” [90]. However, the committee strongly discourages egg or embryo donation to women over age 50 [91].
During their reproductive journeys abroad, infertile patients may face some ethical dilemmas concerning autonomy, dignity, justice, discrimination, patient rights, benefit, and harm. From their own point of view, infertile patients find themselves expecting dignity and autonomy in their quest to have children and that the destination society should respect that. In the countries where same-sex marriage is not allowed, gay couples may seek CBRC to have children. Even in countries where gay marriage is allowed, some couples claim different forms of injustice or discrimination that prevent them from having children although their legal situation is supported by the national law.
In addition, traveling abroad to receive aggressive fertility treatments in a short period of time may result in a higher risk of medical side effects and complications. Traveling patients are often unable to receive standard medical care and second opinions due to time pressures, making them at risk for potential harm [92, 93].
Doctors
A number of fertility treatments are carried out by doctors abroad, including assisted reproduction (IVF, ICSI), third party reproduction (sperm donation, egg donation, embryo donation, surrogacy), PGD, sex selection, and fertility preservation. The main ethical dilemma concerning doctors in the CBRC industry are those related to informed consent and confidentiality [1–3, 78–84].
Informed consent for medical interventions should include the explanation of the procedures, risks, benefits, alternative treatments, and information about the expected outcome and costs. Due to time pressure and less familiarity with medical ethics, doctors abroad may not fulfill the standard criteria of obtaining informed consent from the patients, donors, and surrogates. Many infertile patients may see their doctors abroad for only one visit. In addition, the confidentiality of medical data and security of unused fresh and frozen samples of sperm, eggs, and embryos may not be assured, especially in destinations with poor legal oversight [1–3, 78–84].
Brokers
CBRC brokers or agencies coordinate all involved parties, including infertile patients, doctors, donors, and surrogates. Brokers are also responsible for shipping of sperm, eggs, and embryos when purchased and ordered. Brokers are responsible as well for commodifying and pricing all related medical and non-medical services [94–98].
The main ethical dilemmas concerning brokers are those related to the financial exploitation of donors and surrogates as well as infertile patients. Also, confidentiality and transparency about brokers’ activities may be questionable. Being part of the CBRC industry, brokers or agencies have commercial pressures to provide valid final products, “healthy newborns,” to their customers, “infertile patients,” in return for profit within a certain period of time. Unfortunately, the negative effects of those commercial pressures can be transmitted directly to the women who donate their eggs or serve as surrogates. Examples of these negative effects include limited medical and social care during treatments, after giving birth, or cases of serious maternal or fetal complications. Also, CBRC brokers or agencies may take advantage of the financial needs of poor societies and encourage disadvantaged women to participate in commercial egg donation and surrogacy programs. In these circumstances, brokers may exploit donors and surrogates as well as infertile patients in order to make more profit [94–98].
In fact, brokers are the least transparent party involved in the CBRC industry. There is minimal data available about their legal and financial status, especially in less developed countries. However, it is very easy to find a CBRC broker or agency. A simple internet search can reveal hundreds of their websites worldwide.
Donors and surrogates
Globally, there are several well-known destinations for patients seeking third party reproduction with donors and surrogates. For example, Denmark is known for sperm donation, Spain and Czech Republic are known for egg donation, Belgium is known for embryo donation, while the USA and Russia are known for surrogacy (and India in the past). The main ethical dilemmas concerning donors and surrogates in the CBRC industry are those related to exploitation, benefit and harm, autonomy, child/minor abuse, parental rights, and baby selling [99–105].
Commonly, infertile patients travel to other countries to obtain commercial egg donation or surrogacy. Poverty, illiteracy, and absence of socioeconomic development in many societies around the world may push some disadvantaged women to donate their eggs or serve as surrogates for the financial rewards. Accordingly, the probability of exploitation of poor women in the CBRC industry is very high. Also, these women may suffer from complications of the medical treatments and interventions necessary for egg donation or surrogacy. Complications may be severe or even fatal, including ovarian hyperstimulation syndrome (OHSS), multi-fetal pregnancy, postpartum hemorrhage, and shock [106–110].
Egg donation or surrogacy is a choice that women should be able to make freely, but there is risk for exploitation. The financial stress in poorer societies may make some women feel coerced into the process. Unfortunately, poor women often have poor rights. In countries where the status of women is lower than men, wives, sisters, and daughters can be forced into being donors or surrogates. Some girls are forced to participate in such commercial programs shortly after reaching puberty [106–110].
In many countries, sperm, egg, and embryo donation, as well as surrogacy, is illegal. Therefore, donors and surrogates in such countries have no proper legal protection, and they usually lose their parental rights. In countries where gamete donation and surrogacy are regulated by law, donors and surrogates may have some parental rights; however, these rights are not universal and differ from one country to another. Being known or anonymous usually generates different ethical, social, and religious debates, although gamete donation and surrogacy worldwide are often undertaken anonymously [111].
Pre-implantation genetic diagnosis and sex selection
Sex selection for non-medical reasons is an ongoing ethical debate in many societies worldwide, including the USA. Recently, the ASRM Ethics Committee has published a committee opinion to outline arguments for and against the use of PGD technology for sex selection for non-medical reasons [112].
Fertility preservation
Fertility preservation techniques for medical or non-medical reasons do not usually raise any ethical or social debates except when combined with gamete donation or surrogacy. Other ethical concerns may be generated when experimental procedures are offered to cancer patients, especially to children, such as ovarian or testicular tissue cryopreservation [113, 114]. Recently, the ASRM Ethics Committee has published a comprehensive opinion on fertility preservation and reproduction in cancer patients facing gonadotoxic therapies [115]. In addition, fertility preservation for non-medical reasons such as social egg freezing may raise new social debates in some countries due to cultural reasons.
Religious debates
Religion is an important social institution across the world. As assisted reproductive techniques and third party reproduction (gamete donation and surrogacy) have emerged, they present new dilemmas for different religions. Varying views on the use of these technologies may exist even within the same religion, which creates even more debate than consensus [116–124]. In most religions, such as Christianity, Islam, Judaism, Hinduism, and Buddhism, the conservative religious views completely prohibit assisted reproductive techniques and third party reproduction, while the liberal views partially permit them under certain circumstances. In general, assisted reproductive techniques are allowed by some Christian denominations, Islam (both Sunni and Shia), Judaism, Hinduism, and Buddhism, but often only within the traditional marital relationship. It is important to mention that the Roman Catholic Church still prohibits all types of assisted reproductive techniques and third party reproduction [125–127], even though this techniques are now widely practiced in Catholic-majority countries. In the Muslim world, both Sunni and Shia religious authorities have allowed IVF and ICSI within marriage. However, religious restrictions on third party reproductive assistance in Sunni-majority countries have led some Muslim couples to travel to Shia-majority Iran or Lebanon [128].
Thus, religious views regarding assisted reproduction need to be respected and taken into consideration, especially when fertility treatments are provided to international patients from different cultural backgrounds [129, 130].
Conclusion
CBRC is a growing reality worldwide with potential benefit as well as harm. Therefore, it is critical to evaluate the legal, economic, and ethical issues surrounding CBRC, in order to increase harmonization and reduce any kind of exploitation. For that reason, international organizations have recently begun to collect data and set general guidelines for CBRC. Examples of such international organizations are the European Society of Human Reproduction and Embryology (ESHRE), American Society for Reproductive Medicine (ASRM), International Committee Monitoring Assisted Reproductive Technologies (ICMART), and International Federation of Fertility Societies (IFFS). However, standardization of data collection and establishment of reliable national and global registries are still needed in order to determine the accurate magnitude and scope of CBRC worldwide.
Compliance with ethical standards
Conflict of interest
The authors have declared no conflicts of interest.
Contributor Information
Mahmoud Salama, Phone: +4915733484923, Email: eaims_gm@yahoo.com, http://frauenklinik.uk-koeln.de.
Vladimir Isachenko, Email: v.isachenko@yahoo.com.
Evgenia Isachenko, Email: evgenia.isachenko@uk-koeln.de.
Gohar Rahimi, Email: gohar.rahimi@uk-koeln.de.
Peter Mallmann, Email: peter.mallmann@uk-koeln.de.
Lynn M. Westphal, Email: lynnw@stanford.edu
Marcia C. Inhorn, Email: marcia.inhorn@yale.edu
Pasquale Patrizio, Email: pasquale.patrizio@yale.edu.
References
- 1.Ethics Committee of the American Society for Reproductive Medicine Cross-border reproductive care: an ethics committee opinion. Fertil Steril. 2016;106(7):1627–1633. doi: 10.1016/j.fertnstert.2016.08.038. [DOI] [PubMed] [Google Scholar]
- 2.Pennings G, de Wert G, Shenfield F, Cohen J, Tarlatzis B, Devroey P. ESHRE task force on ethics and law 15: cross-border reproductive care. Hum Reprod. 2008;23(10):2182–2184. doi: 10.1093/humrep/den184. [DOI] [PubMed] [Google Scholar]
- 3.Shenfield F, Pennings G, De Mouzon J, Ferraretti AP, Goossens V, ESHRE Task Force ‘Cross Border Reproductive Care’ (CBRC) ESHRE’s good practice guide for cross-border reproductive care for centers and practitioners. Hum Reprod. 2011;26(7):1625–1627. doi: 10.1093/humrep/der090. [DOI] [PubMed] [Google Scholar]
- 4.Gürtin ZB, Inhorn MC. Introduction: travelling for conception and the global assisted reproduction market. Reprod BioMed Online. 2011;23(5):535–537. doi: 10.1016/j.rbmo.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Inhorn MC, Patrizio P. Rethinking reproductive “tourism” as reproductive “exile”. Fertil Steril. 2009;92(3):904–906. doi: 10.1016/j.fertnstert.2009.01.055. [DOI] [PubMed] [Google Scholar]
- 6.Matorras R. Reproductive exile versus reproductive tourism. Hum Reprod. 2005;20(12):3571. doi: 10.1093/humrep/dei223. [DOI] [PubMed] [Google Scholar]
- 7.Salama M. Cross border reproductive care (CBRC): a global perspective. Obstet Gynecol Int J. 2014;1(2):00008. [Google Scholar]
- 8.Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21(4):411–426. doi: 10.1093/humupd/dmv016. [DOI] [PubMed] [Google Scholar]
- 9.Couture V, Drouin R, Tan SL, Moutquin JM, Bouffard C. Cross-border reprogenetic services. Clin Genet. 2015;87(1):1–10. doi: 10.1111/cge.12418. [DOI] [PubMed] [Google Scholar]
- 10.Ethics Committee of American Society for Reproductive Medicine Cross-border reproductive care: a committee opinion. Fertil Steril. 2013;100(3):645–650. doi: 10.1016/j.fertnstert.2013.02.051. [DOI] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Storrow RF. Assisted reproduction on treacherous terrain: the legal hazards of cross-border reproductive travel. Reprod BioMed Online. 2011;23(5):538–545. doi: 10.1016/j.rbmo.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Van Hoof W, Pennings G. Extraterritorial laws for cross-border reproductive care: the issue of legal diversity. Eur J Health Law. 2012;19(2):187–200. doi: 10.1163/157180912x628226. [DOI] [PubMed] [Google Scholar]
- 14.Crockin SL. Legal perspectives on cross-border reproductive care. Reprod BioMed Online. 2011;23(7):811–813. doi: 10.1016/j.rbmo.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Pennings G. Legal harmonization and reproductive tourism in Europe. Hum Reprod. 2004;19(12):2689–2694. doi: 10.1093/humrep/deh486. [DOI] [PubMed] [Google Scholar]
- 16.Jackson E, Millbank J, Karpin I, Stuhmcke A. Learning from cross-border reproduction. Med Law Rev. 2017;25(1):23–46. doi: 10.1093/medlaw/fww045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Millbank J. Responsive regulation of cross-border assisted reproduction. J Law Med. 2015;23(2):346–364. [PubMed] [Google Scholar]
- 18.Flatscher-Thöni M, Voithofer C. Should reproductive medicine be harmonized within Europe? Eur J Health Law. 2015;22(1):61–74. doi: 10.1163/15718093-12341345. [DOI] [PubMed] [Google Scholar]
- 19.Van Hoof W, Pennings G, De Sutter P. Cross-border reproductive care for law evasion: should physicians be allowed to help infertility patients evade the law of their own country? Eur J Obstet Gynecol Reprod Biol. 2016;202:101–105. doi: 10.1016/j.ejogrb.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 20.Harper J, Geraedts J, Borry P, Cornel MC, Dondorp WJ, Gianaroli L, Harton G, Milachich T, Kääriäinen H, Liebaers I, Morris M, Sequeiros J, Sermon K, Shenfield F, Skirton H, Soini S, Spits C, Veiga A, Vermeesch JR, Viville S, de Wert G, Macek M., Jr ESHG, ESHRE and EuroGentest2. Current issues in medically assisted reproduction and genetics in Europe: research, clinical practice, ethics, legal issues and policy. Hum Reprod. 2014;29(8):1603–1609. doi: 10.1093/humrep/deu130. [DOI] [PubMed] [Google Scholar]
- 21.Harper JC, Geraedts J, Borry P, Cornel MC, Dondorp W, Gianaroli L, Harton G, Milachich T, Kääriäinen H, Liebaers I, Morris M, Sequeiros J, Sermon K, Shenfield F, Skirton H, Soini S, Spits C, Veiga A, Vermeesch JR, Viville S, de Wert G, Macek M, Jr, ESHG; ESHRE; EuroGentest2 Current issues in medically assisted reproduction and genetics in Europe: research, clinical practice, ethics, legal issues and policy. Eur J Hum Genet. 2013;21(Suppl 2):S1–21. doi: 10.1038/ejhg.2013.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crockin SL. Growing families in a shrinking world: legal and ethical challenges in cross-border surrogacy. Reprod BioMed Online. 2013;27(6):733–741. doi: 10.1016/j.rbmo.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 23.International Federation of Fertility Societies IFFS Surveillance 2016. Glob Reprod Health. 2016;1:1–143. [Google Scholar]
- 24.Rashedi AS, SFD R, Ataman LM, Edmonds ME. et al., Survey of fertility preservation options available to patients with cancer around the globe. J Glob Oncol. 2017; 10.1200/JGO.2016.008144. [DOI] [PMC free article] [PubMed]
- 25.Rashedi AS, SFD R, Ataman LM, Edmonds ME, et al. Survey of third-party parenting options associated with fertility preservation available to patients with cancer around the globe. J Glob Oncol. 2017; 10.1200/JGO.2017.009944. [DOI] [PMC free article] [PubMed]
- 26.Forman R. Cross-border reproductive care: a clinician’s perspective. Reprod BioMed Online. 2011;23(7):808–810. doi: 10.1016/j.rbmo.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Spar D. Reproductive tourism and the regulatory map. N Engl J Med. 2005;352(6):531–533. doi: 10.1056/NEJMp048295. [DOI] [PubMed] [Google Scholar]
- 28.Blyth E. Fertility patients’ experiences of cross-border reproductive care. Fertil Steril. 2010;94(1):e11–e15. doi: 10.1016/j.fertnstert.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 29.Whittaker A, Speier A. “Cycling overseas”: care, commodification, and stratification in cross-border reproductive travel. Med Anthropol. 2010;29(4):363–383. doi: 10.1080/01459740.2010.501313. [DOI] [PubMed] [Google Scholar]
- 30.Messinis IE, Messini CI, Daponte A, Garas A, Mahmood T. The current situation of infertility services provision in Europe. Eur J Obstet Gynecol Reprod Biol. 2016. [DOI] [PubMed]
- 31.Hertz R, Nelson MK, Suñol J. Attitudes toward regulations of reproductive care in the European Union: a comparison between travellers for cross-border reproductive care and citizens of the local country. Facts Views Vis Obgyn. 2016;8(3):147–160. [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes EG, Sawyer A, DeJean D, Adamson GD. Cross-border reproductive care in North America: a pilot study testing a prospective data collection program for in vitro fertilization clinics in Canada and the United States. Fertil Steril. 2016;105(3):786–790. doi: 10.1016/j.fertnstert.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 33.Levine AD, Boulet SL, Berry RM, Jamieson DJ, Alberta-Sherer HB, Kissin DM. Assessing the use of assisted reproductive technology in the United States by non-United States residents. Fertil Steril. 2017;108(5):815–821. doi: 10.1016/j.fertnstert.2017.07.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodino IS, Goedeke S, Nowoweiski S. Motivations and experiences of patients seeking cross-border reproductive care: the Australian and New Zealand context. Fertil Steril. 2014;102(5):1422–1431. doi: 10.1016/j.fertnstert.2014.07.1252. [DOI] [PubMed] [Google Scholar]
- 35.Hibino Y, Shimazono Y, Kambayashi Y, Hitomi Y, Nakamura H. Attitudes towards cross-border reproductive care among infertile Japanese patients. Environ Health Prev Med. 2013;18(6):477–484. doi: 10.1007/s12199-013-0345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inhorn MC, Shrivastav P, Patrizio P. Assisted reproductive technologies and fertility “tourism”: examples from global Dubai and the Ivy League. Med Anthropol. 2012;31(3):249–265. doi: 10.1080/01459740.2011.596495. [DOI] [PubMed] [Google Scholar]
- 37.Shenfield F, de Mouzon J, Pennings G, et al. Cross border reproductive care in six European countries. Hum Reprod. 2010;25(6):1361–1368. doi: 10.1093/humrep/deq057. [DOI] [PubMed] [Google Scholar]
- 38.European IVF-monitoring Consortium (EIM); European Society of Human Reproduction and Embryology (ESHRE) Calhaz-Jorge C, De Geyter C, Kupka MS, de Mouzon J, Erb K, Mocanu E, Motrenko T, Scaravelli G, Wyns C, Goossens V. Assisted reproductive technology in Europe, 2013: results generated from European registers by ESHRE. Hum Reprod. 2017;32(10):1957–1973. doi: 10.1093/humrep/dex264. [DOI] [PubMed] [Google Scholar]
- 39.Hudson N, Culley L, Blyth E, Norton W, Pacey A, Rapport F. Cross-border-assisted reproduction: a qualitative account of UK travellers’ experiences. Hum Fertil (Camb) 2016;19(2):102–110. doi: 10.3109/14647273.2016.1168530. [DOI] [PubMed] [Google Scholar]
- 40.Hudson N, Culley L. Assisted reproductive travel: UK patient trajectories. Reprod BioMed Online. 2011;23(5):573–581. doi: 10.1016/j.rbmo.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Van Hoof W, De Sutter P, Pennings G. “Now we feel like we did everything we could”: a qualitative study into the experiences of Dutch patients who travelled to Belgium for infertility treatment. Facts Views Vis Obgyn. 2014;6(4):185–193. [PMC free article] [PubMed] [Google Scholar]
- 42.Van Hoof W, Pennings G, De Sutter P. Cross-border reproductive care for law evasion: a qualitative study into the experiences and moral perspectives of French women who go to Belgium for treatment with donor sperm. Soc Sci Med. 2015;124:391–397. doi: 10.1016/j.socscimed.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 43.Bassan S, Michaelsen MA. Honeymoon, medical treatment or big business? An analysis of the meanings of the term “reproductive tourism” in German and Israeli public media discourses. Philos Ethics Humanit Med. 2013;8:9. doi: 10.1186/1747-5341-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunt J. Cross border treatment for infertility: the counselling perspective in the UK. Hum Fertil (Camb) 2013;16(1):64–67. doi: 10.3109/14647273.2013.770565. [DOI] [PubMed] [Google Scholar]
- 45.Hamilton M. Sperm donation in the United Kingdom in 2010. Hum Fertil (Camb) 2010;13(4):257–262. doi: 10.3109/14647273.2010.518658. [DOI] [PubMed] [Google Scholar]
- 46.Flower D. Assisted reproduction: should egg and sperm donors be paid? J Fam Health Care. 2010;20(2):69–71. [PubMed] [Google Scholar]
- 47.Bay B, Larsen PB, Kesmodel US, Ingerslev HJ. Danish sperm donors across three decades: motivations and attitudes. Fertil Steril. 2014;101(1):252–257.e1. doi: 10.1016/j.fertnstert.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 48.Bergmann S. Reproductive agency and projects: Germans searching for egg donation in Spain and the Czech Republic. Reprod BioMed Online. 2011;23(5):600–608. doi: 10.1016/j.rbmo.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 49.Madero S, Gameiro S, García D, Cirera D, Vassena R, Rodríguez A. Quality of life, anxiety and depression of German, Italian and French couples undergoing cross-border oocyte donation in Spain. Hum Reprod. 2017;32(9):1862–1870. doi: 10.1093/humrep/dex247. [DOI] [PubMed] [Google Scholar]
- 50.Pennings G, de Mouzon J, Shenfield F, Ferraretti AP, Mardesic T, Ruiz A, Goossens V. Socio-demographic and fertility-related characteristics and motivations of oocyte donors in eleven European countries. Hum Reprod. 2014;29(5):1076–1089. doi: 10.1093/humrep/deu048. [DOI] [PubMed] [Google Scholar]
- 51.Ethics Committee of the American Society for Reproductive Medicine Defining embryo donation: an Ethics Committee opinion. Fertil Steril. 2016;106(1):56–58. doi: 10.1016/j.fertnstert.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 52.Sreenivas K, Campo-Engelstein L. Domestic and international surrogacy laws: implications for cancer survivors. Cancer Treat Res. 2010;156:135–152. doi: 10.1007/978-1-4419-6518-9_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pande A. Transnational commercial surrogacy in India: gifts for global sisters? Reprod BioMed Online. 2011;23(5):618–625. doi: 10.1016/j.rbmo.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 54.Saxena P, Mishra A, Malik S. Surrogacy: ethical and legal issues. Indian J Community Med. 2012;37(4):211–213. doi: 10.4103/0970-0218.103466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Covington SN, Patrizio P. Gestational carriers and surrogacy. In: Sauer M, editor. Principles of oocyte and embryo donation. 2. London: Springer Verlag; 2013. pp. 277–288. [Google Scholar]
- 56.Svitnev K. Legal regulation of assisted reproduction treatment in Russia. Reprod BioMed Online. 2010;20(7):892–894. doi: 10.1016/j.rbmo.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 57.Capelouto SM, Archer SR, Morris JR, Kawwass JF, Hipp HS. Sex selection for non-medical indications: a survey of current pre-implantation genetic screening practices among U.S. ART clinics. J Assist Reprod Genet. 2017; 10.1007/s10815-017-1076-2. [DOI] [PMC free article] [PubMed]
- 58.Practice Committee of American Society for Reproductive Medicine Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2013;100(5):1214–1223. doi: 10.1016/j.fertnstert.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 59.Woodruff TK. Oncofertility: a grand collaboration between reproductive medicine and oncology. Reproduction. 2015;150(3):S1–10. doi: 10.1530/REP-15-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salama M, Winkler K, Murach KF, Seeber B, Ziehr SC, Wildt L. Female fertility loss and preservation: threats and opportunities. Ann Oncol. 2013;24(3):598–608. doi: 10.1093/annonc/mds514. [DOI] [PubMed] [Google Scholar]
- 61.Petropanagos A, Cattapan A, Baylis F, Leader A. Social egg freezing: risk, benefits and other considerations. CMAJ. 2015;187(9):666–669. doi: 10.1503/cmaj.141605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.ESHRE Task Force on Ethics and Law. Dondorp W, de Wert G, Pennings G, Shenfield F, Devroey P, Tarlatzis B, Barri P, Diedrich K. Oocyte cryopreservation for age-related fertility loss. Hum Reprod. 2012;27(5):1231–1237. doi: 10.1093/humrep/des029. [DOI] [PubMed] [Google Scholar]
- 63.Rebar RW. Social and ethical implications of fertility preservation. Fertil Steril. 2016;105(6):1449–1451. doi: 10.1016/j.fertnstert.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 64.Bower B, Quinn GP. Fertility preservation in cancer patients: ethical considerations. Adv Exp Med Biol. 2012;732:187–196. doi: 10.1007/978-94-007-2492-1_15. [DOI] [PubMed] [Google Scholar]
- 65.Garceau L, Henderson J, Davis LJ, Petrou S, Henderson LR, McVeigh E, Barlow DH, Davidson LL. Economic implications of assisted reproductive techniques: a systematic review. Hum Reprod. 2002;17(12):3090–3109. doi: 10.1093/humrep/17.12.3090. [DOI] [PubMed] [Google Scholar]
- 66.Collins J. An international survey of the health economics of IVF and ICSI. Hum Reprod Update. 2002;8(3):265–277. doi: 10.1093/humupd/8.3.265. [DOI] [PubMed] [Google Scholar]
- 67.Connolly MP, Ledger W, Postma MJ. Economics of assisted reproduction: access to fertility treatments and valuing live births in economic terms. Hum Fertil (Camb) 2010;13(1):13–18. doi: 10.3109/14647270903401747. [DOI] [PubMed] [Google Scholar]
- 68.Chambers GM, Sullivan E. a, Ishihara O et al. The economic impact of assisted reproductive technology: a review of selected developed countries. Fertil Steril. 2009;91(6):2281–2294. doi: 10.1016/j.fertnstert.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 69.Connolly MP, Hoorens S, Chambers GM. The costs and consequences of assisted reproductive technology: an economic perspective. Hum Reprod Update. 2010;16(6):603–613. doi: 10.1093/humupd/dmq013. [DOI] [PubMed] [Google Scholar]
- 70.Connolly M. Cross-border reproductive care: market forces in action or market failure? An economic perspective. Reprod BioMed Online. 2011;23(7):817–819. doi: 10.1016/j.rbmo.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 71.McKelvey A, David AL, Shenfield F, Jauniaux ER. The impact of cross-border reproductive care or ‘fertility tourism’ on NHS maternity services. BJOG. 2009;116(11):1520–1523. doi: 10.1111/j.1471-0528.2009.02294.x. [DOI] [PubMed] [Google Scholar]
- 72.Austin CR. Legal, ethical and historical aspects of assisted human reproduction. Int J Dev Biol. 1997;41(2):263–265. [PubMed] [Google Scholar]
- 73.Shanner L, Nisker J. Bioethics for clinicians: 26. Assisted reproductive technologies. CMAJ. 2001;164(11):1589–1594. [PMC free article] [PubMed] [Google Scholar]
- 74.Brezina PR, Zhao Y. The ethical, legal, and social issues impacted by modern assisted reproductive technologies. Obstet Gynecol Int. 2012;2012:686253. doi: 10.1155/2012/686253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Frydman PR. Human reproduction: possibilities and ethical borders. Folia Histochem Cytobiol. 2009;47(5):S5–S7. doi: 10.2478/v10042-009-0064-5. [DOI] [PubMed] [Google Scholar]
- 76.Baši M, Milojevi M, Miti D, Cvetkovi J. Ethical aspects in the area of assisted reproduction 2010; 27(3):171–178.
- 77.Inhorn MC. Cosmopolitan conceptions: IVF sojourns in Global Dubai. Durham: Duke University Press; 2015. [Google Scholar]
- 78.Ferraretti AP, Pennings G, Gianaroli L, Natali F, Magli MC. Cross-border reproductive care: a phenomenon expressing the controversial aspects of reproductive technologies. Reprod BioMed Online. 2010;20(2):261–266. doi: 10.1016/j.rbmo.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 79.Crozier GKD, Martin D. How to address the ethics of reproductive travel to developing countries: a comparison of national self-sufficiency and regulated market approaches. Dev World Bioeth. 2012;12(1):45–54. doi: 10.1111/j.1471-8847.2012.00316.x. [DOI] [PubMed] [Google Scholar]
- 80.Deonandan R, Green S, van Beinum A. Ethical concerns for maternal surrogacy and reproductive tourism. J Med Ethics. 2012;38(12):742–745. doi: 10.1136/medethics-2012-100551. [DOI] [PubMed] [Google Scholar]
- 81.Blyth E, Thorn P, Wischmann T. CBRC and psychosocial counselling: assessing needs and developing an ethical framework for practice. Reprod BioMed Online. 2011;23(5):642–651. doi: 10.1016/j.rbmo.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 82.Shalev C, Moreno A, Eyal H, Leibel M, Schuz R, Eldar-Geva T. Ethics and regulation of inter-country medically assisted reproduction: a call for action. Isr J Health Policy Res. 2016;5:59. doi: 10.1186/s13584-016-0117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deonandan R. Recent trends in reproductive tourism and international surrogacy: ethical considerations and challenges for policy. Risk Manag Health Policy. 2015;8:111–119. doi: 10.2147/RMHP.S63862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hammarberg K, Stafford-Bell M, Everingham S. Intended parents’ motivations and information and support needs when seeking extraterritorial compensated surrogacy. Reprod BioMed Online. 2015;31(5):689–696. doi: 10.1016/j.rbmo.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 85.Gossett DR, Nayak S, Bhatt S, Bailey SC. What do healthy women know about the consequences of delayed childbearing? J Health Commun. 2013;18(Suppl 1):118–128. doi: 10.1080/10810730.2013.825677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miura LN, Boxer RS. Women in medicine and the ticking clock. Ann Fam Med. 2013;11(4):381–382. doi: 10.1370/afm.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mac Dougall K, Beyene Y, Nachtigall RD. Age shock: misperceptions of the impact of age on fertility before and after IVF in women who conceived after age 40. Hum Reprod. 2013;28(2):350–356. doi: 10.1093/humrep/des409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wiebe E, Chalmers A, Yager H. Delayed motherhood: understanding the experiences of women older than age 33 who are having abortions but plan to become mothers later. Can Fam Physician. 2012;58(10):e588–e595. [PMC free article] [PubMed] [Google Scholar]
- 89.Corbett SL, Frecker HM, Shapiro HM, Yudin MH. Access to fertility services for lesbian women in Canada. Fertil Steril. 2013;100(4):1077–1080. doi: 10.1016/j.fertnstert.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 90.The Ethics Committee of the American Society for Reproductive Medicine Access to fertility treatment by gays, lesbians, and unmarried persons. Fertil Steril. 2009;92(4):1190–1193. doi: 10.1016/j.fertnstert.2009.07.977. [DOI] [PubMed] [Google Scholar]
- 91.The Ethics Committee of the American Society for Reproductive Medicine Oocyte or embryo donation to women of advanced age: a committee opinion. Fertil Steril. 2013;100(2):337–340. doi: 10.1016/j.fertnstert.2013.02.030. [DOI] [PubMed] [Google Scholar]
- 92.Vincent-Rohfritsch A, Marszalek A, Santulli P, Gayet V, Chapron C, Goffinet F, Le Ray C. Risk of perinatal complication and egg donation: role of resorting to cross-border care? J Gynecol Obstet Biol Reprod (Paris) 2016;45(8):866–875. doi: 10.1016/j.jgyn.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 93.Ahuja KK. Patient pressure: is the tide of cross-border reproductive care beginning to turn? Reprod BioMed Online. 2015;30(5):447–450. doi: 10.1016/j.rbmo.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 94.Casey V, Crooks V. a, Snyder J, Turner L. Knowledge brokers, companions, and navigators: a qualitative examination of informal caregivers’ roles in medical tourism. Int J Equity Health. 2013;12:94. doi: 10.1186/1475-9276-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Speier AR. Brokers, consumers and the internet: how North American consumers navigate their infertility journeys. Reprod BioMed Online. 2011;23(5):592–599. doi: 10.1016/j.rbmo.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 96.Ikemoto LC. Reproductive tourism: equality concerns in the global market for fertility services. Law Ineq. 2009;27:277–309. [Google Scholar]
- 97.Swink DR, Reich B. Outsourcing reproduction : embryos and surrogacy services in the cyberprocreation era. J Health Care Law Policy. 2011;14:241–297. [Google Scholar]
- 98.Penney K, Snyder J, Crooks VA, Johnston R. Risk communication and informed consent in the medical tourism industry: a thematic content analysis of Canadian broker websites. BMC Med Ethics. 2011;12:17. doi: 10.1186/1472-6939-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Burry KA. Reproductive medicine: where we have been, where we are, where are we going? An ethical perspective. Am J Obstet Gynecol. 2007;196:578–580. doi: 10.1016/j.ajog.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 100.Donchin A. Reproductive tourism and the quest for global gender justice. Bioethics. 2010;24(7):323–332. doi: 10.1111/j.1467-8519.2010.01833.x. [DOI] [PubMed] [Google Scholar]
- 101.Schäfer D, Baumann R, Kettner M. Ethics and reproductive medicine. Hum Reprod Update. 1996;2(5):447–456. doi: 10.1093/humupd/2.5.447. [DOI] [PubMed] [Google Scholar]
- 102.Armour KL. An overview of surrogacy around the world: trends, questions and ethical issues. Nurs Womens Health. 2012;16:231–236. doi: 10.1111/j.1751-486X.2012.01734.x. [DOI] [PubMed] [Google Scholar]
- 103.Neri M, Turillazzi E, Pascale N, Riezzo I, Pomara C. Egg production and donation: a new frontier in the global landscape of cross-border reproductive care: ethical concerns. Curr Pharm Biotechnol. 2016;17(4):316–320. doi: 10.2174/1389201017666160118103418. [DOI] [PubMed] [Google Scholar]
- 104.Norton W, Crawshaw M, Hudson N, Culley L, Law C. A survey of UK fertility clinics’ approach to surrogacy arrangements. Reprod BioMed Online. 2015;31(3):327–338. doi: 10.1016/j.rbmo.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 105.Janssens PM, Thorn P, Castilla JA, Frith L, Crawshaw M, Mochtar M, Bjorndahl L, Kvist U, Kirkman-Brown JC. Evolving minimum standards in responsible international sperm donor offspring quota. Reprod BioMed Online. 2015;30(6):568–580. doi: 10.1016/j.rbmo.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 106.The Ethics Committee of the American Society for Reproductive Medicine Consideration of the gestational carrier: a committee opinion. Fertil Steril. 2013;99(7):1838–1841. doi: 10.1016/j.fertnstert.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 107.Loike JD, Fischbach RL. New ethical horizons in gestational surrogacy. J IVF Reprod Med Genet. 2013;1:109. [Google Scholar]
- 108.Brinsden PR. Gestational surrogacy. Hum Reprod Update. 2003;9:483–491. doi: 10.1093/humupd/dmg033. [DOI] [PubMed] [Google Scholar]
- 109.Qadeer I. Social and ethical basis of legislation on surrogacy: need for debate. Indian J Med Ethics. 2009;6(1):28–31. doi: 10.20529/IJME.2009.007. [DOI] [PubMed] [Google Scholar]
- 110.Ergas Y. Babies without borders: human rights, human dignity and the regulation of international commercial surrogacy. Emory Int Law Rev. 2013:1–69.
- 111.Melo-Martín ID. The ethics of anonymous gamete donation: is there a right to know one’s genetic origins? Hast Cent Rep. 2014;44(2):28–35. doi: 10.1002/hast.285. [DOI] [PubMed] [Google Scholar]
- 112.Ethics Committee of the American Society for Reproductive Medicine Use of reproductive technology for sex selection for nonmedical reasons. Fertil Steril. 2015;103(6):1418–1422. doi: 10.1016/j.fertnstert.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 113.Patrizio P, Caplan AL. Ethical issues surrounding fertility preservation in cancer patients. Clin Obstet Gynecol. 2010;53(4):717–726. doi: 10.1097/GRF.0b013e3181f96a70. [DOI] [PubMed] [Google Scholar]
- 114.Cohen CB. Ethical issues regarding fertility preservation in adolescents and children. Pediatr Blood Cancer. 2009;53(2):249–253. doi: 10.1002/pbc.21996. [DOI] [PubMed] [Google Scholar]
- 115.The Ethics Committee of the American Society for Reproductive Medicine Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril. 2013;100(5):1224–1231. doi: 10.1016/j.fertnstert.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 116.Schenker JG. Assisted reproduction practice: religious perspectives. Reprod BioMed Online. 2005;10(3):310–319. doi: 10.1016/s1472-6483(10)61789-0. [DOI] [PubMed] [Google Scholar]
- 117.Nikiforova B. Theological discourse in bioethics: general and confessional differences. Santalka Filosofija. 2006;14(1):62–76. [Google Scholar]
- 118.Sureau C. From transgression to pragmatism in reproductive medicine. Reprod Nutr Dev. 2005;45(3):307–319. doi: 10.1051/rnd:2005023. [DOI] [PubMed] [Google Scholar]
- 119.Fasouliotis SJ, Schenker JG. Social aspects in assisted reproduction. Hum Reprod Update. 1999;5(1):26–39. doi: 10.1093/humupd/5.1.26. [DOI] [PubMed] [Google Scholar]
- 120.Schenker JG. Gender selection: cultural and religious perspectives. J Assist Reprod Genet. 2002;19(9):400–410. doi: 10.1023/A:1016807605886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lanzone A. Ethical issues in human reproduction: catholic perspectives. Gynecol Endocrinol. 2013;29(11):953–954. doi: 10.3109/09513590.2013.825717. [DOI] [PubMed] [Google Scholar]
- 122.Serour GI, Dickens BM. Assisted reproduction developments in the Islamic world. Int J Gynaecol Obstet. 2001;74(2):187–193. doi: 10.1016/s0020-7292(01)00425-8. [DOI] [PubMed] [Google Scholar]
- 123.Serour GI. Ethical issues in human reproduction: Islamic perspectives. Gynecol Endocrinol. 2013;29(11):949–952. doi: 10.3109/09513590.2013.825714. [DOI] [PubMed] [Google Scholar]
- 124.Schenker JG. Human reproduction: Jewish perspectives. Gynecol Endocrinol. 2013;29(11):945–948. doi: 10.3109/09513590.2013.825715. [DOI] [PubMed] [Google Scholar]
- 125.Markwell HJ, Brown BF. Bioethics for clinicians: 27. Catholic bioethics CMAJ. 2001;165(2):189–192. [PMC free article] [PubMed] [Google Scholar]
- 126.Serour GI, Aboulghar MA, Mansour RT. Bioethics in medically assisted conception in the Muslim world. J Assist Reprod Genet. 1995;12(9):559–565. doi: 10.1007/BF02212574. [DOI] [PubMed] [Google Scholar]
- 127.Schenker JG. Assisted reproductive technology: perspectives in Halakha (Jewish religious law) Reprod BioMed Online. 2008;17(Suppl 3):17–24. doi: 10.1016/s1472-6483(10)60326-4. [DOI] [PubMed] [Google Scholar]
- 128.Inhorn MC. The new Arab man: emergent masculinities, technologies, and Islam in the Middle East. Princeton, NJ: Princeton University Press; 2012. [Google Scholar]
- 129.Inhorn MC. Local babies, global science: gender, religion, and in vitro fertilization in Egypt. New York: Routledge; 2003. [Google Scholar]
- 130.Inhorn MC, Patrizio P. The global landscape of cross-border reproductive care: twenty key findings for the new millennium. Curr Opin Obstet Gynecol. 2012;24(3):158–163. doi: 10.1097/GCO.0b013e328352140a. [DOI] [PubMed] [Google Scholar]