Figure 7.
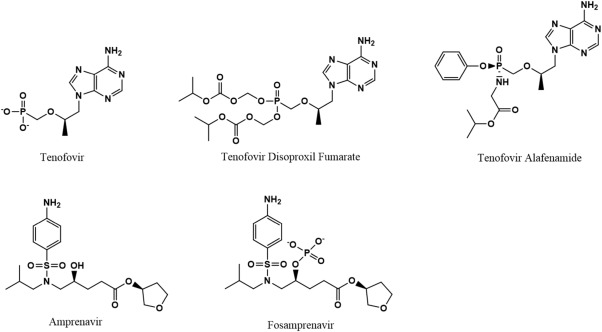
Chemical structures of TFV and APV and their respective prodrugs. The conjugation of acyloxyalkyl esters or aryls to TFV increase its lipophilicity and the addition of l‐alanine isopropyl ester enhances accumulation of the active drug in HIV target cells. The addition of a phosphate group to APV improves its water solubility.
