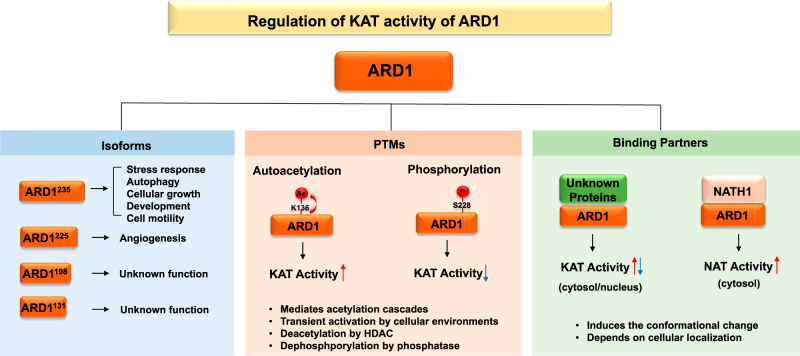
Fig. 2. Regulation of the KAT activity of ARD1.
The KAT activity of ARD1 is regulated by PTMs. While autoacetylation at the K136 residue stimulates the KAT activity of ARD1, phosphorylation at the S228 residue inhibits the KAT activity of ARD1. Autoacetylation is conserved among ARD1 isoforms, including ARD1235 and ARD1225; however, autoacetylation regulates differential cellular functions depending on the isoform. While the autoacetylation of ARD1235 enhances cellular growth, the autoacetylation of ARD1225 inhibits angiogenesis. The KAT activity of ARD1 might also be regulated by unknown binding proteins