Abstract
Kratom is an herbal compound that has been used as a recreational drug though is not regulated by the Food and Drug Administration. We report a 19-year-old male with recurrent seizures that developed during daily Kratom abuse as a self-treatment for anxiety. Following recurrent focal impaired awareness seizures in addition to generalized tonic–clonic seizures, he was begun on anti-seizure drugs. Seizures subsided after completing rehabilitation. Brain MRI at 29 months revealed bilaterally symmetric T1-hyperintensity in globus pallidus, subthalamic nuclei, and cerebral peduncles. Our case suggests Kratom abuse may be associated with structural brain lesions on MRI and symptomatic focal epilepsy.
Abbreviations: ASD, anti-seizure drugs; DEA, Drug and Enforcement Administration; FDA, Food and Drug Administration; GTC, generalized tonic–clonic
Keywords: Kratom, Abuse, Epilepsy, MRI, Dependency, Opioid
Highlights
-
•
Kratom has become a popular recreational drug in the West.
-
•
Kratom works as a stimulant but at high doses it has sedative/anti-nociceptive effects.
-
•
Kratom abuse may be associated with structural brain lesions on MRI.
-
•
No standard methods exist to detect Kratom or its metabolites on drug screening.
1. Introduction
Kratom is an herbal supplement (leaves of extracts(s) of the Mitragyna speciosa tree in the coffee family) that originated in Southeast Asia where it is chewed or ingested as a tea to either give energy or curb anxiety. In small doses, Kratom works as a stimulant, but in higher-doses it has sedative and anti-nociceptive effects [1]. Oftentimes, supplements are taken to fortify or maintain health and are believed to be innocuous [2]. There is evidence that most supplements do not prevent disease or death, and their use is not justified when they have no clear benefit because they could be harmful [3]. Since the 1990s, Kratom has become a popular recreational drug in the West. This is largely due to migration of people from Asia to the U.S. and from the dissemination of information through internet marketing [4]. Its true prevalence of usage has remained unclear due to limited reports. In 2016, several million consumers were reported purchasing Kratom from greater than 10,000 retail locations in the U.S. with an estimated annual market of 207 million dollars [5]. Anecdotal reports have included side effects such as seizure, hypothyroidism, hepatotoxicity, and coma [1], [6], [7], [8]. In a case report, Kratom has shown to produce reversible injury to the posterior white matter of the brain [9]. We report a patient with chronic monotherapy Kratom use who developed focal epilepsy.
2. Case report
A 19-year-old right-handed Caucasian male with anxiety was without risks factors for epilepsy. He was evaluated after experiencing a first seizure. He was initially found down at school, in the bathroom with post-event confusion and was suspected to have experienced an unwitnessed generalized tonic–clonic (GTC) seizure. He was concurrently being treated with intermittent lisdexamfetamine dimesylate usage for attention deficit hyperactivity disorder. A brain MRI performed shortly after the first seizure was reported to reveal no focal abnormalities. A complete metabolic profile, blood count, urine drug screen, and electroencephalogram were within normal limits. He was not treated with anti-seizure drugs (ASDs).
One year later, the patent experienced a second seizure characterized by awakening from sleep in a transient “dream-like reverie state,” with generalized muscle soreness and tongue laceration. At this time, the patient admitted to excessive use of Kratom (several pills per day) for several months to self-treat anxiety. Typically, Kratom dosage can vary between 2 to 8 g, producing stimulant to sedative effects, respectively. Further, ASDs were not administered for a suspected provoked seizure.
After a third focal seizure, he was prescribed levetiracetam (LEV), 500 mg twice daily. Despite treatment, a witnessed focal to bilateral GTC seizure occurred 21 months after the first event, despite taking LEV. The event was initially described as “blacking out” with disorientation and lip smacking prior to the GTC seizure. At this time, the patient admitted to continued use of Kratom. A urine drug screen returned negative. Lisdexamfetamine dimesylate and intermittent use of alprazolam had been discontinued, but he continued taking Kratom as an affordable and available means of alleviating anxiety and providing him with energy. After his fifth GTC seizure, he admitted to being non-compliant with LEV due to changes in mood and was switched to lamotrigine for its mood stabilizing effects.
The sixth GTC seizure resulted in a motor vehicle accident. In addition to Kratom use, he then admitted to intermittent use of marijuana (once weekly), lisdexamfetamine dimesylate use (3 times weekly), rare alprazolam use (monthly), and intermittent alcohol consumption (4 drinks weekly). He followed up with Psychiatry and due to chronic Kratom abuse was recommended to drug rehabilitation.
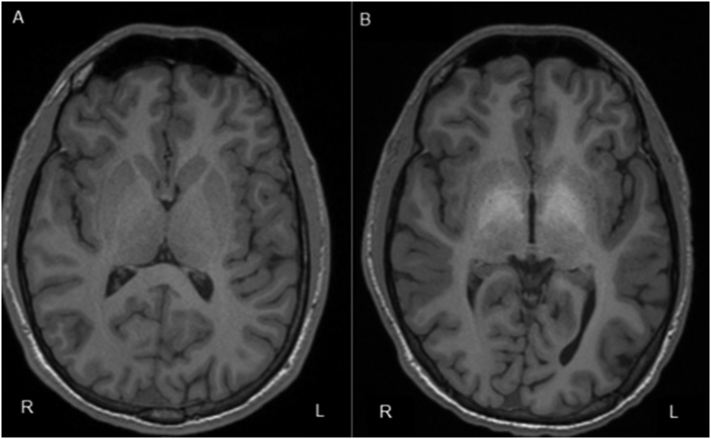
After switching to lamotrigine and discontinuing Kratom the patient was seizure-free. Nonetheless, breakthrough seizures were noted when a relapse of Kratom abuse occurred. A brain MRI performed 29 months after the initial seizure revealed bilateral symmetric T1 hyperintensity in the diencephalon including the globus pallidus, subthalamic nuclei, and portions of the cerebral peduncles (Fig. 1). Repeat metabolic profiles were within normal limits. After successful completion of a substance abuse rehabilitation program, no further seizures were reported on lamotrigine. A follow-up brain MRI was sought to visualize if structural brain abnormalities had subsided since discontinuation of Kratom, but the patient was lost to follow-up when insurance changed.
Fig. 1.
A. Brain MRI, transverse T1-weighted demonstrating normal imaging after first seizure event; B. Brain MRI, 3-T high-resolution, 29 months after initial seizure event demonstrating increased signal in the globus pallidus bilaterally during chronic Kratom use.
3. Discussion
Herbal supplements are used for their perceived benefits without much consideration given to harmful properties. They are used globally and the prevalence of usage continues to rise. Like patients abusing illicit drugs, our patient abusing Kratom benefited from detoxification at a supervised facility with trained medical professionals to monitor and provide medical support during the process. Kratom use in the U.S. has received limited attention yet the active ingredients are alkaloid substances called mitragynine and 7-hydroxymitragynine, and are mediated via the monoaminergic and opioid (mu- and kappa-) receptors [10]. These mechanisms of action are similar to opioids partly due to its molecular structure referred to as biased agonist [4]. Given the current worldwide opioid analgesic crisis and potential for misuse, abuse, and dependence, Kratom may be subject to recreational abuse.
There are a number of herbal supplements that are associated with seizures including Black cohosh [11], Bearberry [12], Ma Huang [13], kava-kava [12], Yohimbe [14], and Monkshood [12]. On the other hand, herbal supplements have had putative anti-seizure effects, though the lack of evidence precludes this conclusion [15]. For example, exogenous cannabinoids can mimic the endogenous system and may have a role in reducing seizure frequency or protect against neurodegeneration [16]. Devinsky et al. performed an open-label trial in patients between ages 1–30 with severe, drug-resistant childhood-onset seizures [17]. Oral cannabidiol 2–5 mg/kg/day and titrated to a maximum tolerated dose of 25 mg/kg or 50 mg/kg/day (depending on the center) reduced seizure frequency and was safe in children and young adults, though randomized controlled trials like the trial of cannabindiol in Dravet's syndrome [18] are necessary to validate these findings in adults with focal seizures.
Between 1983 and 1989, National Poisons Unit in London reported 5131 inquiries. Of these, 968 were related to herbal supplements, and of which, 245 (25%) were symptomatic [12]. Side effects arising from herbal supplements may be due to various reasons, such as, misidentifying the plant species, overlooking the toxicity of the plant, variability in the chemical constituents of the herb, adulteration, incorrect dosing, and differing potency depending on the conditions in which the plant was grown [19].
Currently, no standard methods exist to detect Kratom or its metabolites on drug screening after ingestion and this is a contributing factor to its abuse potential. Like our patient, this also complicates the clinical scenario for the treating physician and delays management [10]. Evidence also suggests high rates of dependency, development of withdrawal symptoms, and craving among chronic users [20]. In one study, more than half of regular Kratom users (> 6 months) developed severe withdrawal symptoms while 45% demonstrated moderate dependency [20]. Consequently, withdrawal symptoms tend to worsen with chronic use. In a retrospective review in Thailand, 52 Kratom cases were identified of which 76.9% resulted in Kratom poisoning and 23.1% were due to withdrawal [21]. Symptoms commonly reported were palpitations in 22.5% and seizures in 17.5%. An infant born to a chronic Kratom-abusing mother within this cohort experienced withdrawal symptoms and emphasized the role of transplacental transmission [21]. Currently, no specific treatment regimens for Kratom withdrawal or addiction exist.
Increasing Kratom abuse in the U.S. has created disputes regarding public safety. In parallel to the opioid crisis, the U.S. Drug and Enforcement Administration (DEA) listed Kratom as a “Drug of Concern” in 2008 [22]. Several states have banned the sale of Kratom [5]. In 2016, the DEA announced temporary placement of Kratom into a Schedule I category governed by the Controlled Substances Act [22]. However, public rebuttals, a bipartisan response from the U.S. congress, and arguments made by the American Kratom Association resulted in the DEA to withdraw its proposition [4]. Consequently, the DEA, with input from the Food and Drug Administration (FDA) and National Institute on Drug Abuse undertook a full abuse potential assessment of Kratom use to develop regulatory recommendations [5]. The conclusion was Kratom did not appear to be of a public health threat and emergency scheduling of this supplement or any of its specific alkaloids was considered insignificant [4]. Additionally, it was suggested that those individuals who consume Kratom for reasons other than recreational use may resort to illicit unregulated Kratom venders, thus exposing them to additional risk [4]. Since the banning of its importation by the FDA, its availability has flown under the radar and has increased. It is now being sold in powder and tablet form at tobacco stores, and marketed in shops, purchased online, or mixed into drinks.
4. Conclusion
We associate structural brain abnormality on MRI and symptomatic focal epilepsy with chronic Kratom abuse during initial use and again during relapse. Thus far, complications from Kratom abuse have received limited attention and untoward behavioral dependence and complications have been largely anecdotal. Kratom abuse is likely to increase due to market globalization and readily available internet-driven supply chains, in addition to the relative safety of Kratom being endorsed as a “natural” herbal supplement. The accessibility and unknown safety profile of Kratom calls for urgent safety assessment to help guide appropriate regulatory control.
Footnotes
Declaration of interest: None.
References
- 1.Boyer E.W., Babu K.M., Adkins J.E., McCurdy C.R., Halpern J.H. Self-treatment of opioid withdrawal using kratom (Mitragynia speciosa korth) Addiction. 2008;103(6):1048–1050. doi: 10.1111/j.1360-0443.2008.02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey R.L., Gahche J.J., Miller P.E., Thomas P.R., Dwyer J.T. Why US adults use dietary supplements. JAMA Intern Med. 2013;173(5):355–361. doi: 10.1001/jamainternmed.2013.2299. [DOI] [PubMed] [Google Scholar]
- 3.Guallar E., Stranges S., Mulrow C., Appel L.J., Miller E.R., III Enough is enough: stop wasting money on vitamin and mineral supplements. Ann Intern Med. 2013;159(12):850–851. doi: 10.7326/0003-4819-159-12-201312170-00011. [DOI] [PubMed] [Google Scholar]
- 4.Henningfield J.E., Fant R.V., Wang D.W. The abuse potential of kratom according the 8 factors of the controlled substances act: implications for regulation and research. Psychopharmacology. 2018;235(2):573–589. doi: 10.1007/s00213-017-4813-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Economic impact of Kratom scheduling. Botanical Education Alliance; 2016. [Google Scholar]
- 6.Kapp F.G., Maurer H.H., Auwarter V., Winkelmann M., Hermanns-Clausen M. Intrahepatic cholestasis following abuse of powdered kratom (Mitragyna speciosa) J Med Toxicol. 2011;7(3):227–231. doi: 10.1007/s13181-011-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelsen J.L., Lapoint J., Hodgman M.J., Aldous K.M. Seizure and coma following Kratom (Mitragynina speciosa Korth) exposure. J Med Toxicol. 2010;6(4):424–426. doi: 10.1007/s13181-010-0079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheleg S.V., Collins G.B. A coincidence of addiction to “Kratom” and severe primary hypothyroidism. J Addict Med. 2011;5(4):300–301. doi: 10.1097/ADM.0b013e318221fbfa. [DOI] [PubMed] [Google Scholar]
- 9.Castillo A., Payne J.D., Nugent K. Posterior reversible leukoencephalopathy syndrome after kratom ingestion. Proc (Baylor Univ Med Cent) 2017;30(3):355–357. doi: 10.1080/08998280.2017.11929647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warner M.L., Kaufman N.C., Grundmann O. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Int J Legal Med. 2016;130(1):127–138. doi: 10.1007/s00414-015-1279-y. [DOI] [PubMed] [Google Scholar]
- 11.Shuster J. Herbal remedies and seizures. Nursing. 1997;27(4):75. doi: 10.1097/00152193-199704000-00038. [DOI] [PubMed] [Google Scholar]
- 12.Bateman J., Chapman R.D., Simpson D. Possible toxicity of herbal remedies. Scott Med J. 1998;43(1):7–15. doi: 10.1177/003693309804300104. [DOI] [PubMed] [Google Scholar]
- 13.Cupp M.J. Herbal remedies: adverse effects and drug interactions. Am Fam Physician. 1999;59(5):1239–1245. [PubMed] [Google Scholar]
- 14.D'Arcy P.F. Adverse reactions and interactions with herbal medicines. Part 1. Adverse reactions. Adverse Drug React Toxicol Rev. 1991;10(4):189–208. [PubMed] [Google Scholar]
- 15.Tyagi A., Delanty N. Herbal remedies, dietary supplements, and seizures. Epilepsia. 2003;44(2):228–235. doi: 10.1046/j.1528-1157.2003.19902.x. [DOI] [PubMed] [Google Scholar]
- 16.Kolikonda M.K., Srinivasan K., Enja M., Sagi V., Lippmann S. Medical marijuana for epilepsy? Innov Clin Neurosci. 2016;13(3–4):23–26. [PMC free article] [PubMed] [Google Scholar]
- 17.Devinsky O., Marsh E., Friedman D., Thiele E., Laux L., Sullivan J. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15(3):270–278. doi: 10.1016/S1474-4422(15)00379-8. [DOI] [PubMed] [Google Scholar]
- 18.Devinsky O., Cross H.J., Laux L., Marsh E., Miller I., Nabbout R. Trial of cannabidiol for drug-resistant seizures in the dravet syndrome. N Engl J Med. 2017;376(21):2011–2020. doi: 10.1056/NEJMoa1611618. [DOI] [PubMed] [Google Scholar]
- 19.Huxtable R.J. The harmful potential of herbal and other plant products. Drug Saf. 1990;5(Suppl. 1):126–136. doi: 10.2165/00002018-199000051-00020. [DOI] [PubMed] [Google Scholar]
- 20.Singh D., Muller C.P., Vicknasingam B.K. Kratom (Mitragyna speciosa) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol Depend. 2014;139:132–137. doi: 10.1016/j.drugalcdep.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Trakulsrichai S., Tongpo A., Sriapha C., Wongvisawakorn S., Rittilert P., Kaojarern S. Kratom abuse in Ramathibodi Poison Center, Thailand: a five-year experience. J Psychoactive Drugs. 2013;45(5):404–408. doi: 10.1080/02791072.2013.844532. [DOI] [PubMed] [Google Scholar]
- 22.Schedules of controlled substances: placement of mitragynine and 7-Hydroxymitragynine into schedule I. Federal Register: U.S. drug enforcement administration. 2016. https://www.federalregister.gov/documents/2016/08/31/2016-20803/schedules-of-controlled-substances-temporary-placement-of-mitragynine-and-7-hydroxymitragynine-into Available from: [PubMed]