A 70-year-old female visited our hospital due to a 5-year history of long-standing persistent atrial fibrillation (LSPsAF) refractory to medical treatment. Chest x-ray and echocardiography were free of abnormalities and did not detect manifest structural heart disease. A hybrid AF procedure (Epicardial thoracoscopic ablation + appendage resection, and endocardial catheter ablation) was referred to patient.
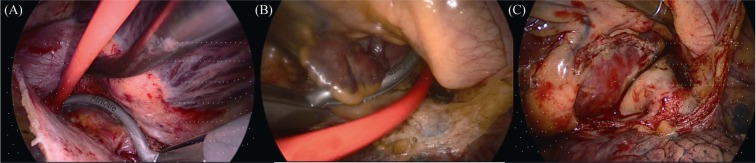
First step procedure (epicardial thoracoscopic ablation + appendage resection) started on the right side with placement of endoscopic tools after right lung deflation, the pericardium was opened anterior to the phrenic nerve (PN) from the superior vena cava (SVC) to the diaphragm and gently separated and secured with three stitches. The interatrial groove was dissected with endoscopic scissors as well as the access to the oblique sinus between the inferior vena cava (IVC) and the right lower pulmonary vein (PV). An articulating and illuminating dissector (LumiTip Dissector; AtriCure, OH) was used to surround the right PVs to guide insertion of the ablation clamp (Isolator Synergy; AtriCure, OH). A series of ablations (usually 5–10) around right PVs were performed followed by verification of an entry block. A roof line and bottom line (connecting the superior and inferior bilateral PVs, respectively) were performed using a linear pen device (Bipolar Linear Pen; AtriCure, OH). The left-side approach was similar to the right side. The vein and ligament of Marshall was dissected using scissors and both ends were burned with the linear pen. The Lumitip with the rubber tourniquet was used to place the bipolar clamp and a series of ablations around left PVs were made until entry block was documented. Ganglionated plexi were located and ablated, guided by high-frequency stimulation and vagal response inducing bradycardia (Figure 1 A, B). The left atrial appendage was excluded using an Articulating Endoscopic Linear Cutter (Echelonflex 45, Ethicon, CA) (Figure 1 C). At this time, the patient presented paroxysmal atrial flutter.
Figure 1. Epicardial thoracoscopic ablation and LAA resection.
(A): Right PVs were clamped by ablation clamp from right-side approach; (B): left PVs were clamped by ablation clamp from left-side approach; (C): LAA stub after resection. LAA: left atrial appendage; PVs: pulmonary veins.
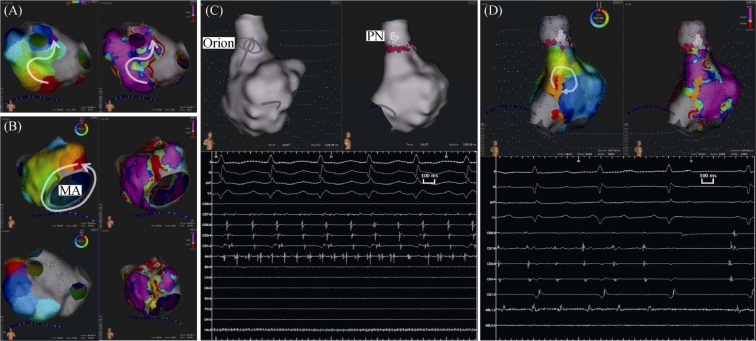
The second step (endocardial catheter ablation), under fluoroscopy guiding, a 10-polar catheter was introduced into the coronary sinus (CS) via left femoral vein and an 8.5 Fr SL1 long sheath (St. Jude Medical, USA) was introduced into the left atrium (LA) via right femoral vein after trans-septal puncture. Anatomical reconstruction of the LA and PVs was created with Rhythmia™ mapping system (Boston Scientific, USA) and 64-pole basket IntellaMap Orion mapping catheter (Boston Scientific, USA). Normal sinus rhythm was present at the beginning of the procedure, activation and voltage maps were simultaneously generated during CS ostium pacing. Radio-frequency (RF) energy (30–35 W at 43 °C with 17 mL/min flow) was applied at conduction gap (rigid of left inferior PV) from above-mentioned epicardial lesions was ablated endocardially with a 3.5 mm cooled tip RF catheter (Triguy™, APT Medical, PRC). (Figure 2A) After achieving left inferior PV isolation, posterior LA wall was mapped to confirm electrical isolation.
Figure 2. Conduction gap detection, AT induction and endocardial catheter ablation.
(A): conduction gap at rigid of left inferior PV during CS ostium pacing-rhythm; (B): LA activation mapping present counter clock-wise activation around MA during AT1 and ablation lesion was set at LA anterior wall; (C): SVC firing was detected and PN was marked, then ablation was performed for SVC isolation; (D): further RA activation mapping, micro re-entry activation pattern was present at RA lower crista terminalis during AT2. And AT2 was terminated soon during ablation. AT: atrial tachycardia; CS: coronary sinus; LA: left atrium; MA: mitral annuls; PN: phrenic nerve; PV: pulmonary vein; RA: right atrium; SVC: superior vena cava.
Furthermore, incremental atrial pacing (protocol up to 300 bpm) was performed to detect atrial tachycardia (AT). An AT1 with tachycardia cycle-length (TCL) 246 ms was induced, and then detailed LA activation mapping present counter clock-wise activation around mitral annuls. Entrainments were performed at distal and proximal CS catheter site with post pacing interval (PPI) 270 ms and 248 ms, respectively. RF energy was applied at slow conduction area (also low voltage area) of LA anterior wall, then AT1 was terminated soon (Figure 2B). After re-induction, An AT2 with TCL 220 ms was induced, entrainment at distal and proximal CS present very long PPI. Sequentially, we determined to perform right atrium (RA) map. SVC firing was detected and PN was marked, then RF energy was applied for SVC isolation (Figure 2C), but AT2 activation sequence and TCL were unchanged. Further RA activation mapping, micro re-entry activation pattern was present at RA lower crista terminalis with good entrainment response. AT2 was terminated soon during ablation at target site (Figure 2D). Further stimulation can't induce AT or AF any more, then RF ablation of the cavotricuspid isthmus was performed according to protocol. Non-PV trigger of AF can't be detected by isoproterenol (4 µg/min for 5 min) and a bolus of high-dose adenosine (60 mg). The whole procedure was completed without any complications. The post-operation electrocardiogram and echocardiography of the patient were normal. At 12-month follow-up, the patient still maintained sinus rhythm (SR) and without any evidence for AT or AF recurrence.
To our best knowledge, this is the first case of hybrid procedure using automated ultra-high density mapping system. The efficacy and safety of hybrid ablation seems to be very promising.[1],[2] The Rhythmia™ mapping system could automatically achieve ultra-high density electro-anatomy information and markedly improve signal resolution during activation/substrate mapping.[3] The novel mini-basket catheter, which incorporated small unidirectional electrodes to suppress noise and far field signals,[4] was more sensitive than the traditional LASSO catheter in detecting PV potentials and atrium substrate.[5] That would enable a better understanding of different arrhythmogenic substrate, such as epicardial thoracoscopic ablation lesion, surgical resection scar and intrinsic low voltage/slow conduction area.[6]
Acknowledgments
This work was supported by National Nature Science Foundation of China (No. 81370295), Science and Technology Program of Guangdong Province, China (No. 2017A020215054), Science and Technology Planning of Guangzhou City, China (No. 2014B070705005).
References
- 1.Bulava A, Mokracek A, Hanis J, et al. Correlates of arrhythmia recurrence after hybrid epi- and endocardial radiofrequency ablation for persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2017;10:e005273. doi: 10.1161/CIRCEP.117.005273. [DOI] [PubMed] [Google Scholar]
- 2.Pison L, La Meir M, van Opstal J, et al. Hybrid thoracoscopic surgical and transvenous catheter ablation of atrial fibrillation. J Am Coll Cardiol. 2012;60:54–61. doi: 10.1016/j.jacc.2011.12.055. [DOI] [PubMed] [Google Scholar]
- 3.Latcu DG, Bun SS, Viera F, et al. Selection of critical isthmus in scar-related atrial tachycardia using a New Automated Ultrahigh Resolution Mapping System. Circ Arrhythm Electrophysiol. 2017;10:e004510. doi: 10.1161/CIRCEP.116.004510. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa H, Ikeda A, Sharma T, et al. Rapid high resolution electroanatomical mapping: evaluation of a new system in a canine atrial linear lesion model. Circ Arrhythm Electrophysiol. 2012;5:417–424. doi: 10.1161/CIRCEP.111.968602. [DOI] [PubMed] [Google Scholar]
- 5.Schaeffer B, Hoffmann BA, Meyer C, et al. Characterization, mapping, and ablation of complex atrial tachycardia: initial experience With a novel method of ultra high-density 3D mapping. J Cardiovasc Electrophysiol. 2016;27:1139–1150. doi: 10.1111/jce.13035. [DOI] [PubMed] [Google Scholar]
- 6.Anter E, McElderry TH, Contreras-Valdes FM, et al. Evaluation of a novel high-resolution mapping technology for ablation of recurrent scar-related atrial tachycardias. Heart Rhythm. 2016;13:2048–2055. doi: 10.1016/j.hrthm.2016.05.029. [DOI] [PubMed] [Google Scholar]