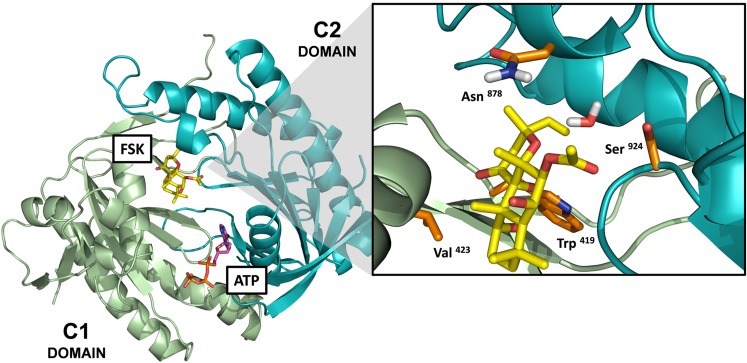
Fig. 6.
Location of mutated residues on AC1 constructs within the forskolin-binding site. Model of the catalytic domains of AC1 based on the crystal structure of the C1(AC5)-C2(AC2) catalytic domains (PDB: 1AZS) (Tesmer et al., 1997). The model includes the ATP and forskolin (FSK) molecules bound at their pseudosymmetric binding sites within the interface of the C1-C2 domains. The enlarge panel corresponds to the forskolin-binding site, and the side chains of the mutated amino acids are represented in orange sticks. The amino acid numbering corresponds to the numbering of the full amino acid sequence for human AC1. *In the 1AZS crystal structure, the Asn878 residue corresponds to Lys897 on the C2 domain of AC2.