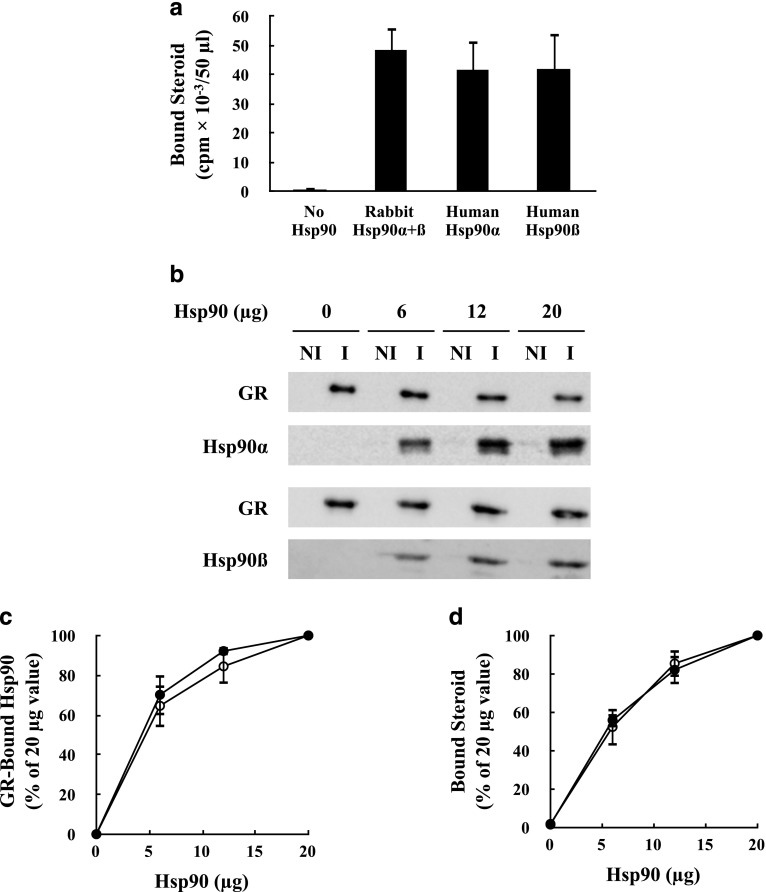
Fig. 1.
Human Hsp90α and Hsp90β have the same activity at generating GR steroid binding. (a) Stripped GR immunopellets were incubated for 20 minutes at 30°C with Hsp70, Hop, Hsp40, and p23 in the presence of 20 μg of rabbit Hsp90α and Hsp90β or human Hsp90α or human Hsp90β. Immune pellets were washed and steroid binding was assayed. (b) Stripped GR immunopellets were incubated with Hsp70, Hop, Hsp40, and p23 in the presence of the indicated amounts of human Hsp90α or Hsp90β. After washing, the pellets were divided in half with one half being assayed for GR•Hsp90 heterocomplex formation by Western blot and the other half being assayed for steroid binding. Immunopellets prepared with immune (I) anti-GR or with nonimmune (NI) antibodies. The GR and Hsp90 bands were scanned and the amount of Hsp90 bound was expressed relative to the 20-μg value at 100%. (c) and (d) Comparison of the relative amounts of GR•Hsp90 heterocomplex and steroid binding, respectively. (●) Hsp90α; (○) Hsp90β. The values for (a), (c), and (d) represent the mean ± S.D. for three experiments.