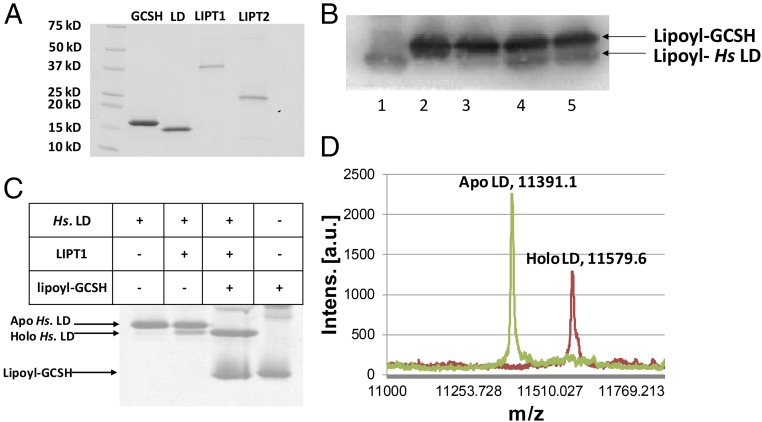
Fig. 3.
LIPT1 catalyzes transfer of lipoyl moieties from GCSH to an LD derived from human pyruvate dehydrogenase. (A) Purification of the lipoate assembly proteins. The proteins were purified as described in Experimental Procedures and analyzed by SDS/PAGE on 4–20% polyacrylamide gels. The molecular weights of the Bio-Rad broad-range protein standards are indicated. (B) Western blot analysis of SDS/PAGE assay of LIPT1 lipoyl amidotransferase activity using anti-lipoyl antibody and B. subtilis LplJ-generated lipoyl-GCSH as the substrate. Lipoyl-GCSH was synthesized with LplJ plus ATP and lipoic acid and then purified using anion exchange chromatography to remove LplJ and residual ATP. Lanes: 1, standard lipoyl-LD prepared by LplJ modification of human LD (Hs apo-LD); 2, lipoyl-GCSH; 3, lipoyl-GCSH plus the human LD; 4 and 5, lipoyl-GCSH incubated with LIPT1 and LD. (C) LIPT1 lipoyl amidotransferase activity analyzed by urea gel electrophoresis. Loss of the positive charge of the modified lysine ϵ-amino group of the LD results in faster migration of the modified form on these gels. The gel was stained with Coomassie Blue. (D) Electrospray mass-spectrometric analysis of lipoylated human LD and the remaining apo LD from the reaction of gel (B, lane 4). The calculated difference in mass (delta mass) between the apo and lipoyl forms was 188, whereas the observed delta mass was 188.5. Note that, in A and B, trace levels of LD lipoylation were seen in the absence of GCSH, which is attributed to LIPT1-bound lipoyl-AMP that survives purification and crystallization (28). The traces of lipoylation that appears without LIPT1 in B seems likely to be due the lipoate assembly pathway of the wild-type E. coli strain used for protein production.