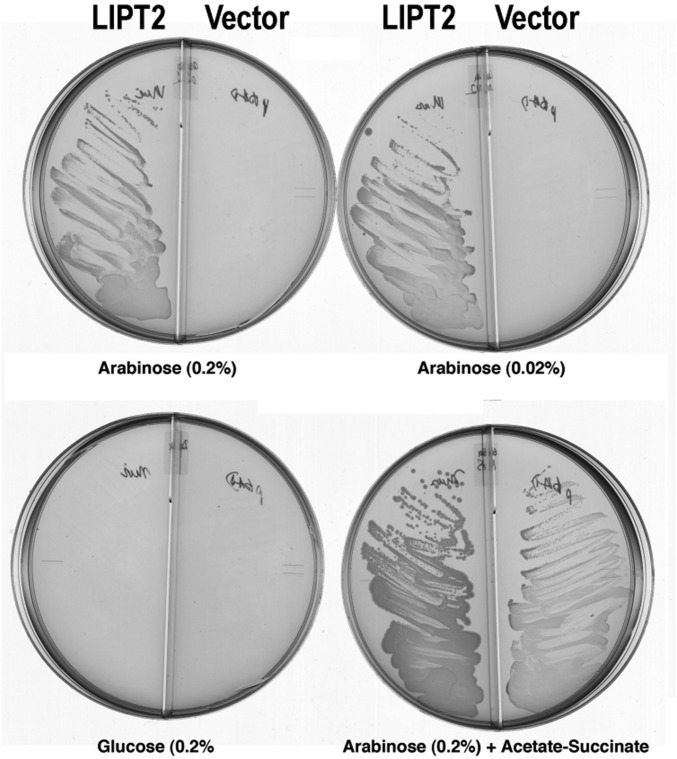
Fig. 6.
Complementation of the lipB deletion mutation (∆lipB) of E. coli strain QC145 by expression of mouse LIPT2. A synthetic gene encoding the full-length mouse LIPT2 coding sequence was inserted into plasmid vector pBAD322A, which provided the transcription and translation sequences required for expression of the protein to give plasmid pCY754. Transcription was from the vector araBAD promoter, which is induced by arabinose and repressed by glucose. The plasmid was introduced into strain QC145 (∆lipB::cml ∆lplA::kan) (68), which carries a deletion of the lplA lipoate ligase gene in addition to the ∆lipB deletion. The lplA mutation was included because mutant LplA proteins can bypass the ∆lipB defect by scavenging cellular octanoic acid (34). The medium was M9 minimal salts with glycerol as carbon source (glycerol allows basal expression of the araBAD promoter) and 100 μg/mL sodium ampicillin to select for plasmid maintenance. Derivatives of strain QC145 carrying either the LIPT2 plasmid pCY754 or the empty vector were grown on glucose minimal salts containing 5 mM each of sodium acetate and sodium succinate, which bypasses the lipoylation requirement. These cultures were then streaked onto glycerol minimal salts plates supplemented with arabinose, glucose, or acetate plus succinate as given on the figure. The plates used are divided into sectors by plastic walls to prevent cross-feeding. The left sector of each plate contained the strain with the LIPT2 plasmid pCY754, whereas the right sector contained the empty pBAD322A vector. No growth was seen on plates that contained only glycerol (basal expression). The plates were incubated for 2 d at 37 °C.