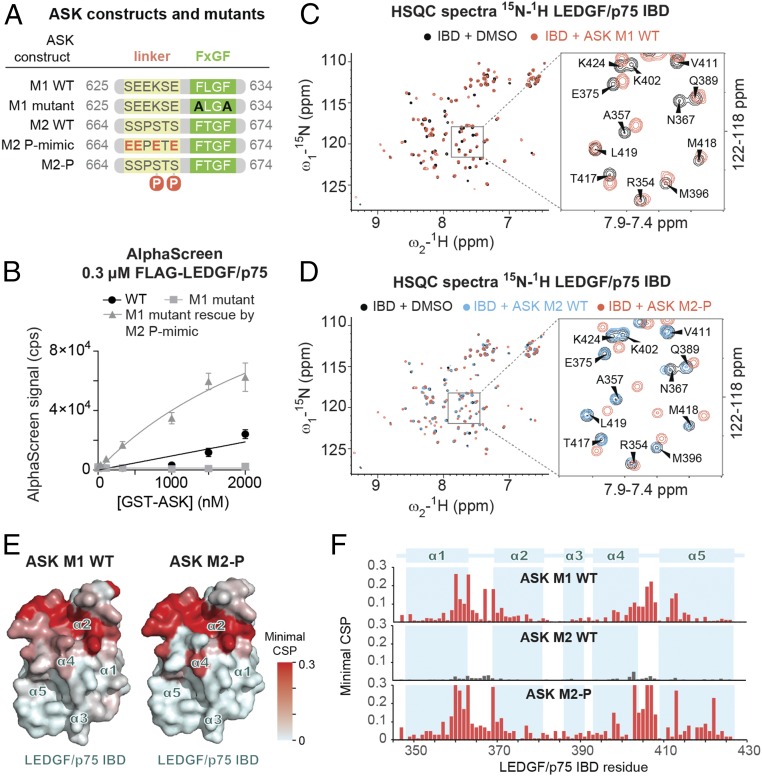
Fig. 5.
ASK has two IBMs whose affinities for the LEDGF/p75 IBD are differentially regulated by phosphorylation. (A) Diagrams of WT and modified constructs of ASK motif 1 and 2 (M1, M2) used in B–E. (B) The WT and/or mutated recombinant ASK protein constructs were titrated against FLAG-tagged LEDGF/p75 in AlphaScreen. Error bars represent SDs calculated from independent measurements (n = 4). (C and D) Comparison of the NMR 2D 15N/1H HSQC spectra of the 15N-labeled IBD in the absence and presence of different ASK synthetic peptides (see also SI Appendix, Fig. S8). (E) IBD amino acid residues that are significantly perturbed upon addition of ASK M1 and phosphorylated ASK M2 (as determined by NMR spectroscopy) are highlighted in red on the surface of the IBD structure. (F) Representation of the minimal CSP in backbone amide signals of the IBD upon addition of ASK-derived peptides.