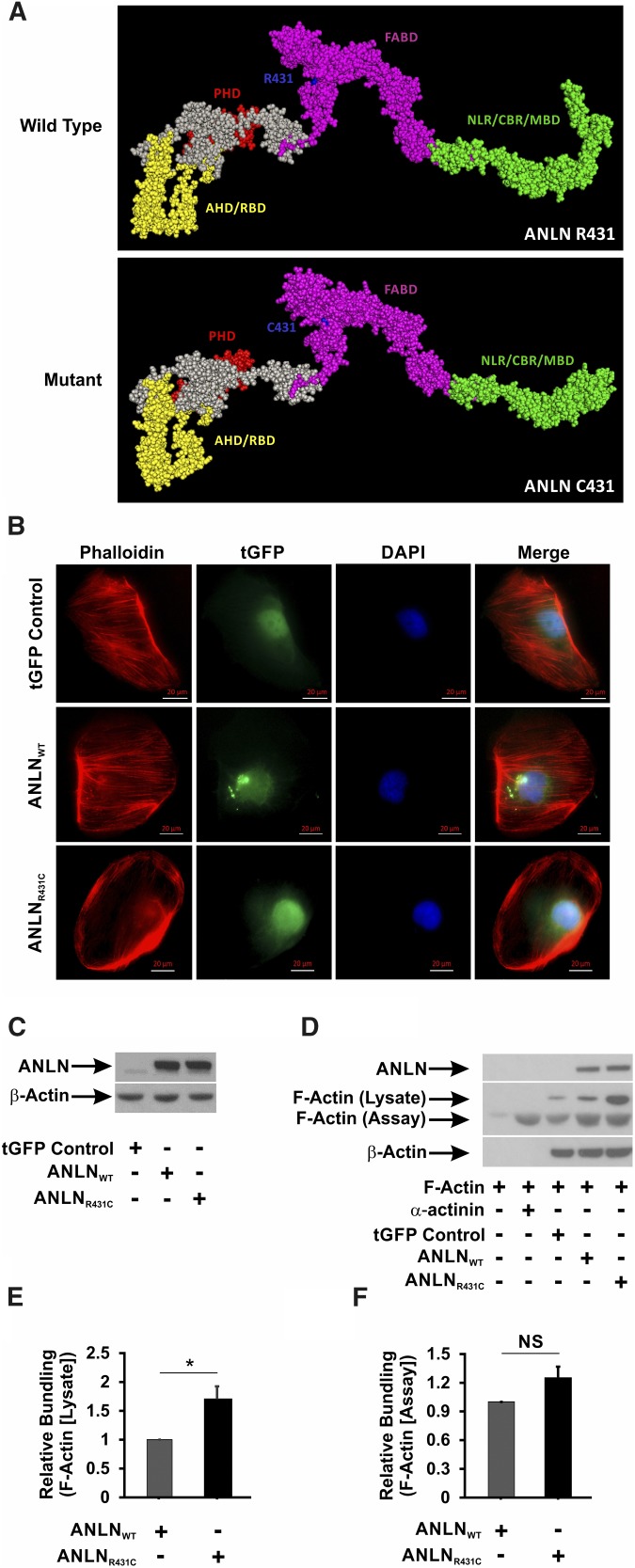
Figure 2.
The R431C mutation distorts the secondary structure and F-actin bundling activity of ANLN. (A) In silico modeling of the effect of R431C mutation on the secondary structure of ANLN reveals that the introduction of a cysteine residue at amino acid position 431 distorts the architecture of the F-actin binding domain (FABD) and the CD2-associated protein binding region (CBR). AHD, Anillin Homology Domain; MBD, Myosin Binding Domain; NLR, Nuclear Localization Region; PHD, Pleckstrin Homology Domain; RBD, Rho A Binding Domain. (B) ANLNWT and ANLNR431C cells were stained with a Phalloidin antibody to visualize F-actin. GFP-tagged ANLN expression can be seen in the nucleus of the three lines. Phalloidin staining showed F-actin fibers extending from end to end in regular array throughout the cell in the ANLNWT cell line compared with ANLNR431C-overexpressing podocytes that showed a paucity of F-actin staining, with small aggregates present mostly in the periphery. (C) Western blot analysis of lentiviral-infected GFP control, turbo GFP (tGFP)–tagged ANLNWT, and tGFP-tagged ANLNR431C immortalized podocyte cell lines for ANLN and β-actin protein levels. Both experimental lines displayed similar levels of tGFP-ANLN, whereas endogenous ANLN is visible in a lower band. (D and E) Actin bundling/polymerization assays were performed with or without the addition of assay-specific nonmuscle F-actin. Transfected HEK 293 cell lines overexpressing ANLNR431C displayed increased bundling/polymerization of lysate-associated F-actin (upper band) compared with the wild-type ANLN (P=0.03). (D and F) There was no difference in relative bundling/polymerization of assay-specific purified F-actin (lower band) between the two ANLN cell lines (P=0.08). *P=0.03.