Figure 5.
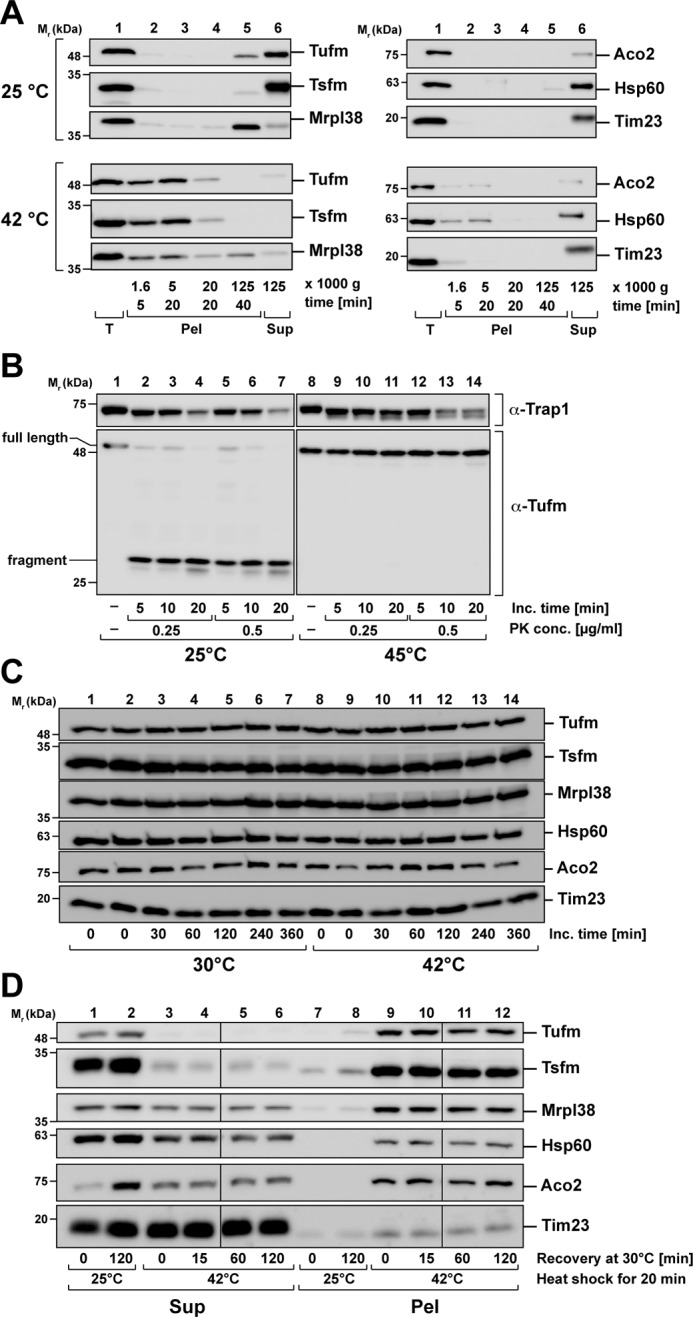
Nature of Tufm aggregates after heat stress. A, analysis of aggregate size after heat stress. Isolated mitochondria were incubated for 20 min under stress (42 °C) and control (25 °C) conditions. Protein aggregates were fractionated by differential centrifugation (supernatants of each centrifugation step were transferred to the next centrifugation step). B, protease resistance of Tufm aggregates. Isolated mitochondria were incubated for 20 min at the indicated temperatures. Mitochondria were lysed with 0.5% Triton X-100, and the extracts were treated with the indicated concentrations of proteinase K. The resulting proteolytic fragments of Tufm and a matrix control protein (Trap1) were detected by SDS–PAGE and Western blotting. C, degradation of mitochondrial proteins during heat stress. Intact energized mitochondria were incubated for up to 6 h at 30 °C (control) and 42 °C (heat stress). Protein was precipitated with TCA and analyzed by SDS–PAGE and Western blotting using the indicated antibodies. D, recovery of aggregation after heat shock. Isolated mitochondria were heat-stressed at 42 °C for 20 min and afterward incubated for up to 2 h at 30 °C. During the recovery incubation, mitochondria were energized and supplied with ATP. Supernatants (Sup) and pellets (Pel) were separated by centrifugation and analyzed by SDS–PAGE and Western blotting.
