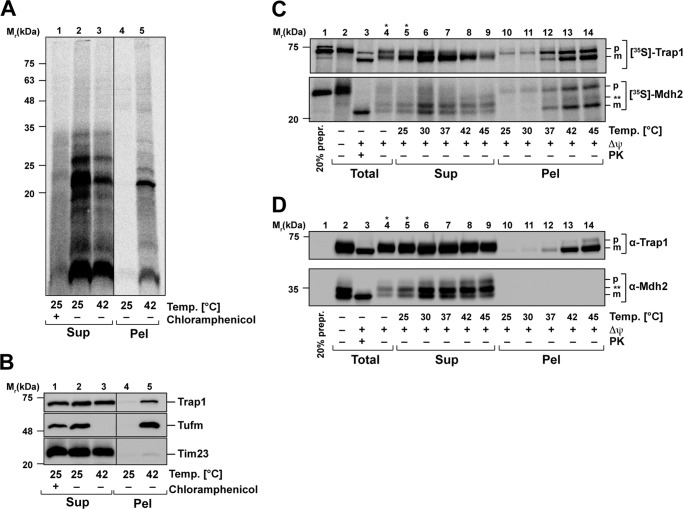
Figure 7.
Heat-induced aggregation of nascent mitochondrial polypeptides. A, heat stress of fresh synthesized mitochondria-encoded proteins. Newly translated mitochondrial proteins were labeled with [35S]Met/Cys under normal conditions (30 °C). Control samples were treated with chloramphenicol to impede mitochondrial translation. Directly after the translation reaction, mitochondria were heat-treated for 20 min at the indicated temperatures and lysed, and aggregates were separated by centrifugation as described. Supernatants (Sup) and pellets (Pel) were analyzed by SDS–PAGE, Western blotting, and autoradiography. B, samples prepared as described in A were analyzed using the indicated antisera. C, heat-induced aggregation of newly imported proteins. After in vitro import of [35S]Met/Cys-labeled preproteins Trap1 and Mdh2 as described in Fig. 6C, mitochondria were heat-stressed for 20 min at the indicated temperatures. After lysis, aggregates were separated by centrifugation, and supernatants (Sup), pellets (Pel), and total amounts were analyzed by SDS–PAGE, Western blotting, and autoradiography. D, samples prepared as described in C were analyzed using the indicated antisera. Δψ, inner membrane potential; PK, proteinase K treatment; p, precursor; m, mature protein. Asterisks indicate lanes where less sample was loaded.