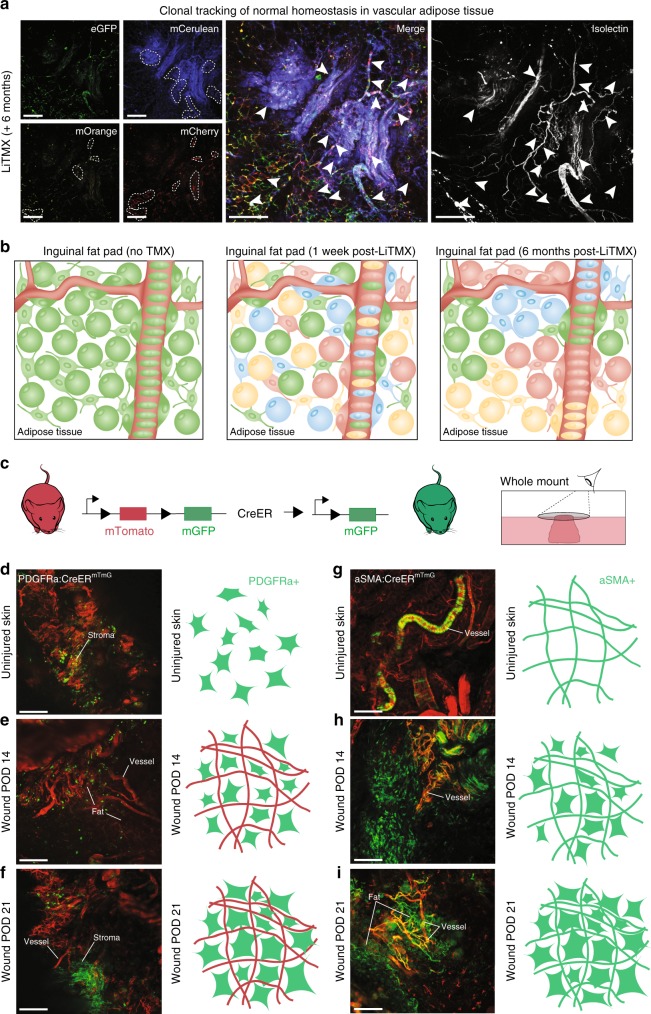
Fig. 4.
LiTMX application for tracing vasculogenic lineages. a Clonal tracking of adipose tissue involved with vasculature in the inguinal fat pad in Rainbow mice at 6 months after LiTMX application (left panel shows split channels, middle panel—merged). Isolectin stains cells engaged with vasculature (right panel). White dotted lines (left) and arrowheads (middle and right) mark clonal cell populations. b Schematic illustrating the fat pad prior to LiTMX application (left panel), 1 week after application with LiTMX-mediated recombination labeling individual cells (middle panel), and the expansion of clonal populations in uninjured fat pad tissue 6 months after induction (right panel). c–i Schematic of experimental application of LiTMX to uninjured skin in PDGFRα-CreERT2::Rosa26-mTmG (left panels, d–f) and αSMA-CreERT2::Rosa26-mTmG (right panels, g–i) mice: c Schematic of the mTmG mouse construct. d, g LiTMX labeling results in highly-specific localized activation of cells engaged with fibrous tissue and vasculature, respectively, in uninjured skin at homeostasis. (White labeling of wound structures as indicated, confocal images at left in each panel, schematics at right highlight induced cellular phenotypes based on the Cre drivers used). e, h At POD 14 after cutaneous wounding and LiTMX induction, the PDGFRα-CreERT2::Rosa26-mTmG construct highlights activated fibrogenic cells involved in wound healing, while the αSMA-CreERT2::Rosa26-mTmG construct highlights stromal cells associated with vasculature. f, i At POD 21 after cutaneous wounding, the cells seen at POD 14 (e, h), clonally expand. Experiments conducted with n = 3 replicates per timepoint (where applicable) per condition (unless otherwise indicated), 2 dorsal wounds per mouse (where applicable), scale bars represent 200 μm (unless otherwise indicated)