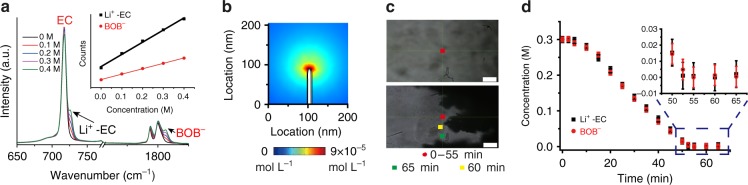
Fig. 2.
Experimental and simulation results for validating “charge neutrality”. a The spontaneous Raman spectra of LiBOB / (TEGDME: EC v/ 7:3) electrolyte with a concentration from 0 M to 0.4 M. Inset is the plot of counts at the designated wavenumber (725 cm–1 for Li+-EC solvent solvation and 1830 cm–1 for BOB–) versus the concentration of LiBOB. b COMSOL finite element simulation on a 10 nm wide electrode tip during lithium reduction to show that the concentration difference between the cation and anion is less than 0.09 mM. A zoomed-out image can be found in Supplementary Fig. 2. c Optical images of the Li electrode at the beginning and end of lithium electrodeposition. The colored squares show positions where the Raman spectra were taken, which is near a lithium dendrite tip. Corresponding Raman spectra and voltage profile can be found in Supplementary Fig. 3. White scale bars are 50 μm. d The concentration changes in Li+ and BOB– vs. time at the locations and times in c. The average difference between [Li+] and [BOB–] at 16 points is well below 2 mM at a noise level of 8.3 mM for Li+-EC solvent solvation and 5.8 mM for BOB–