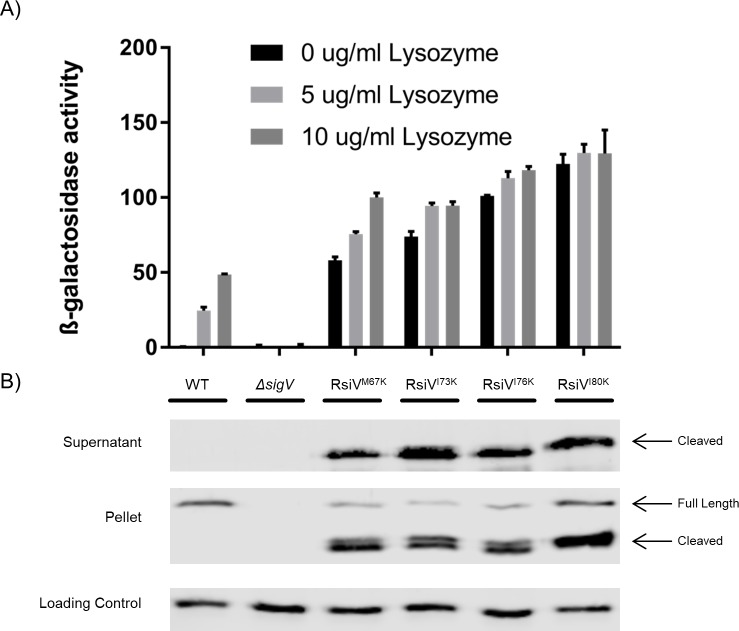
Fig 3. Lysine substitutions in the amphipathic helices lead to constitutive σV activity.
A. Various hydrophobic residues of the amphipathic helices were substituted with lysine residues to disrupt the charge of the amphipathic helix (M67K, I73K, I76K, I80K). These constructs were measured for σV activity using a PsigV-lacZ reporter and a β-galactosidase assay. This experiment was done in triplicate and standard deviation is represented by error bars. B. The lysine substitution constructs (M67K, I73K, I76K, I80K) were further analyzed by western blot to measure RsiV degradation. Cells were grown to mid log. The pellet and supernatants were collected and samples were analyzed by western blot with α-RsiV59-258 antibodies. Streptavidin IR680LT was used detect PycA which served as a loading control [67]. The color blot showing both pellet and loading control on a single gel is S5 Fig.