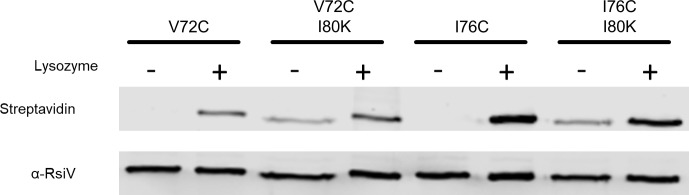
Fig 6. Lysine substitution disrupts the membrane association of hydrophobic residues.
To determine the effect of lysine substitution on hydrophobic residue accessibility in the amphipathic helices we created a construct with N-term 6xHis to purify RsiV and A66W to allow for the purification of RsiV exposed to lysozyme. The cysteine mutations (V72C and I76C) were combined with an I80K substitution to create the constructs RsiVV72C I80K and RsiVI76C I80K. Cells were grown to mid log and the SCAM assay was performed. The resulting purified products were analyzed by western blot with Streptavidin IR680LT to detect the MPB label and α-RsiV59-258 antibodies to serve as a loading control.