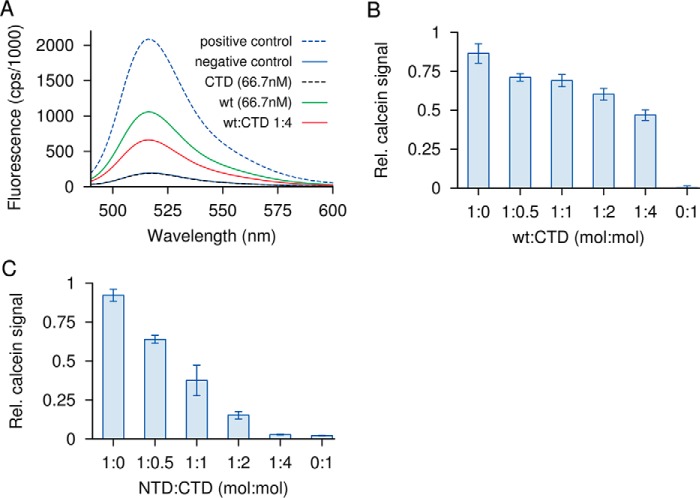
Figure 3.
Permeabilization of liposomes by CAMP factor or its fragments and inhibitory activity of the C-terminal domain. Liposomes containing phosphatidylcholine, cholesterol, and phosphatidylglycerol were loaded with self-quenching concentrations of calcein. Membrane permeabilization releases and dilutes calcein, which increases its fluorescence. Detergent permeabilization is used as a positive control. A, intact CAMP factor (wt) at 66.7 nm causes half-maximal permeabilization. The CTD alone has no effect; its addition to WT at a 4-fold molar excess partially inhibits the latter. B, the same experiment plotted using a relative scale for calcein release, with additional molar ratios for CTD/WT included. C, NTD at 1 μm causes a similar extent of membrane permeabilization as a 15-fold lower amount of WT. NTD too is subject to inhibition by CTD. Error bars, S.E.