Figure 2.
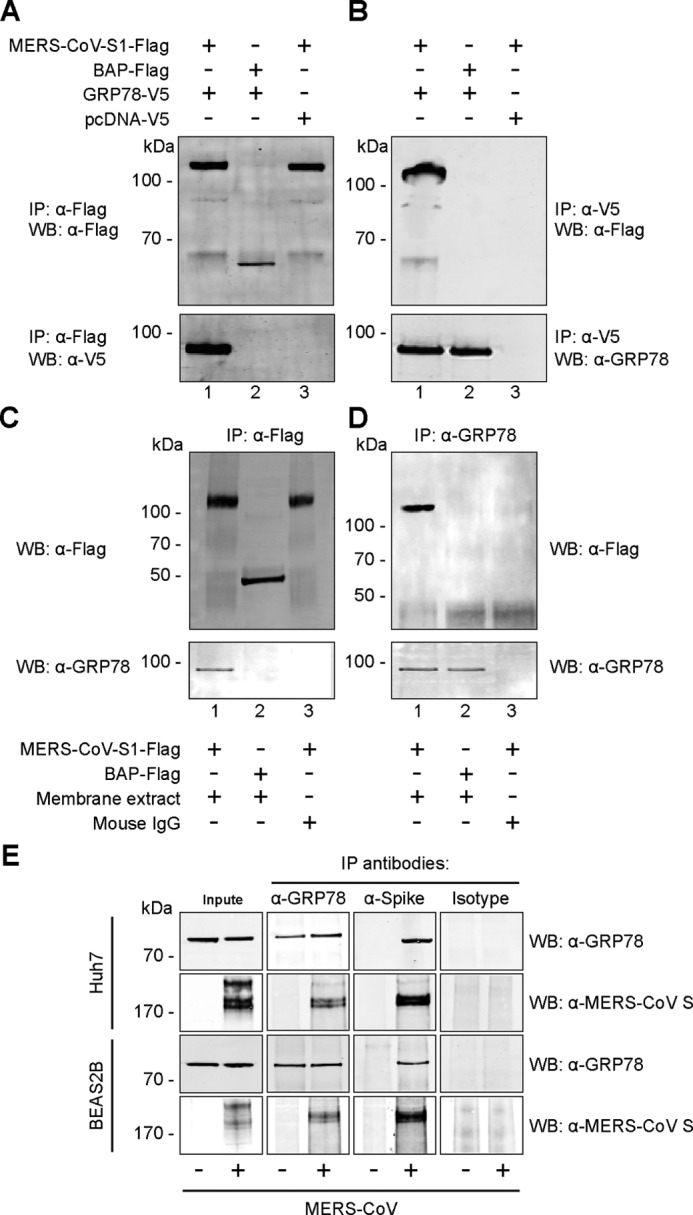
GRP78 interacts with the MERS-CoV spike. A, BHK21 cells were transfected with pcDNA–GRP78–V5 (lanes 1 and 2) or empty vector (lane 3). The cell lysate was immunoprecipitated (IP) with either purified recombinant MERS-CoV–S1–FLAG protein (lanes 1 and 3) or E. coli bacterial alkaline phosphatase (BAP)-FLAG protein (lane 2) pre-adsorbed onto anti-FLAG M2-agarose beads. The precipitated protein complex was detected using the anti-FLAG antibody or the anti-V5 antibody. B, reciprocal co-IP was performed using GRP78 as the bait protein. Purified MERS-CoV–S1–FLAG (lanes 1 and 3) or BAP–FLAG proteins (lane 2) were immunoprecipitated with overexpressed GRP78–V5 or pcDNA–V5 proteins pre-adsorbed on anti-V5 Sepharose beads. The precipitated protein complex was detected using the anti-FLAG antibody or the anti-GRP78 antibody. C, membrane fraction of Huh7 cells was extracted and immunoprecipitated with either MERS-CoV–S1–FLAG(lanes 1 and 3) or BAP–FLAG (lane 2). D, reciprocal co-IP was performed using GRP78 as the bait. Mouse IgG was used in place of the membrane extract as a negative control. E, endogenous co-IP was performed in MERS-CoV- or mock-infected Huh7 and BEAS2B cells. Immunoprecipitation was performed using the anti-GRP78 antibody, the anti-MERS-CoV spike antibody, or the mouse isotype control. The precipitated protein complexes were detected with the anti-MERS-CoV spike antibody or the anti-GRP78 antibody. WB, Western blotting.
