Figure 2.
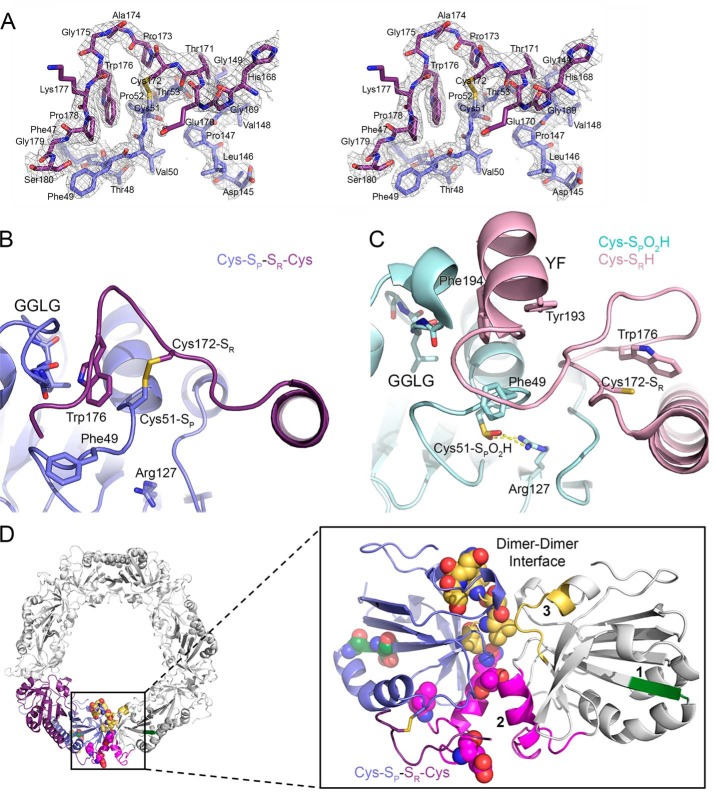
Crystal structures of Prx2 help to identify regions implicated in hyperoxidation. A, stereoview of the 2Fo − Fc map contoured at the 1 σ level (chains E and F), including the Cys-SP–SR-Cys disulfide bond between Prx2 subunits. B, active-site structure of oxidized Prx2, Prx2-SPSR. The new structure reveals the necessary reorganization of the active site for disulfide bond formation. In this locally unfolded conformation, the C terminus is present as a coil that positions Trp176 (purple subunit) so that it packs into a crevice generated by the disulfide bond and the GGLG motif (blue subunit). Importantly, Trp176 is >20 Å away from this position in the Prx2-SPO2H structure as shown in the next panel. C, active-site structure of hyperoxidized Prx2 (chains A and B). In this fully folded conformation, the Cys51-SPO2H moiety (cyan) has an electrostatic interaction with Arg127 (Protein Data Bank code 1QMV) (35). The C terminus of the adjacent subunit (pink) places the Cys172-SR residue ∼14 Å away from the Cys51-SP residue. The YF motif (residues 193 and 194) is present in a helix that packs against the GGLG motif. D, summary of structural changes between Prx oxidation states. Left, one Prx dimer is highlighted in its decameric context (other Prx dimers are colored white). Right, location of regions that have the potential to influence the resistance to hyperoxidation. One subunit of the Prx dimer is shown in reference to the adjacent subunit (white) across the dimer-dimer interface of the toroid. Regions 1–3 are shown in green, magenta, and yellow, respectively. Region 1 is located within the β-strand and turn that precede the helix on which the Cys51-SP residue is located. Regions 2 and 3 interact with the same regions on the adjacent subunit. The residues denoted by spheres are those that differ in sequence between Prx1 and Prx2 (see Fig. 3).