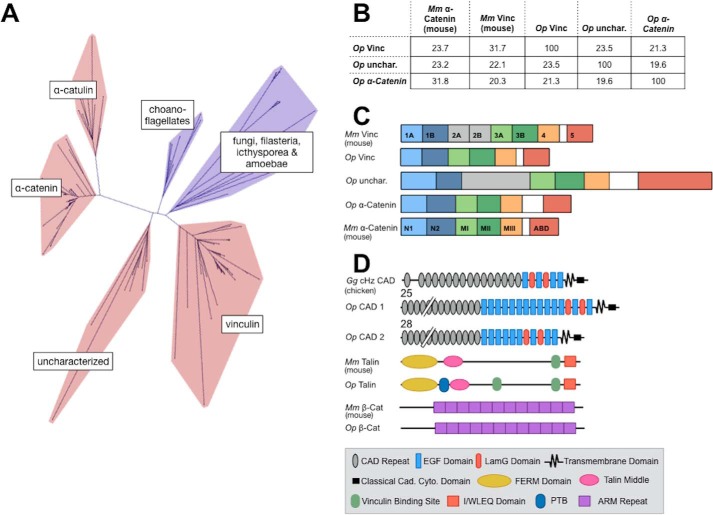
Figure 1.
VIN homology proteins of the sponge, O. pearsei. A, unrooted network depicting the phylogeny of VIN-family proteins (red, sequences from animal species; blue, sequences from nonanimal species). Three VIN-family proteins from O. pearsei were found to fall within the animal-exclusive clades corresponding to vinculin, α-catenin, and a functionally uncharacterized clade. A detailed phylogeny is provided in Figs. S1–S7. B, the percentage of identity of amino acid sequences of Mus musculus αE-catenin (Mm α-cat), Mm vinculin (Mm Vinc), and Op VIN proteins. C, domain schematics of Mm αE-catenin, Mm vinculin, and Op VIN proteins. Mm vinculin is composed of seven 4-helix bundle domains (D1–D4), a proline-rich hinge region (white), and a C-terminal 5-helix bundle (D5, the actin-binding domain). Domains 1, 2, and 3 each comprise two 4-helix bundles that share a central, long helix and are therefore subdivided into subdomains A and B. Mm αE-catenin shares a similar structure to Mm vinculin but lacks domain D2 (gray); the N-terminal domain is homologous to vinculin D1, and the M domain comprises three 4-helix bundles equivalent to vinculin D3A, D3B, and D4. The homologous domains in Op vinculin, Op uncharacterized, and Op α-catenin are color-coded. The length of the domain schematics represents the length of the corresponding amino acid sequences. D, Pfam-predicted domain composition of potential binding partners of Op VIN proteins examined in this study, shown relative to their vertebrate counterparts. Predicted Pfam domains of Op CAD1, Op CAD2, Op Talin, and Op β-catenin are annotated in the figure legend. The numbers above Op CAD1 and Op CAD2 indicate the number of extracellular cadherin repeats.