Figure 2.
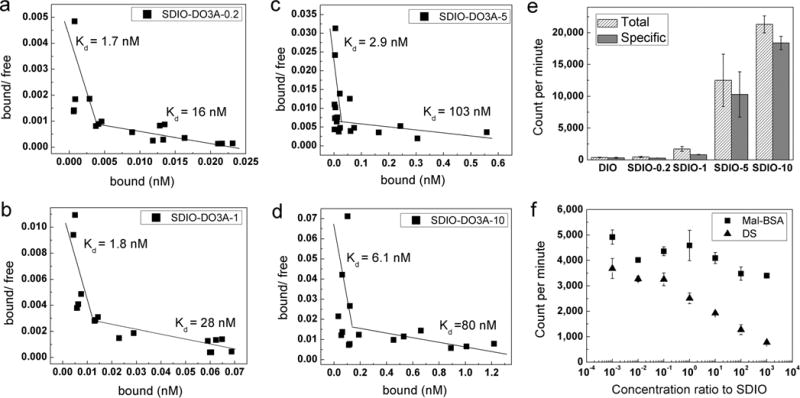
111In3+-radiolabeled nanoparticle binding studies at 4 °C. (a–d) Scatchard plots of SDIO–DO3A nanoparticles of four sulfation levels binding to SR-A on cell surfaces. The biphasic plots observed for all of the sulfated nanoparticles suggested two different binding sites on the receptors with different affinities. (DIO–DO3A can barely bind to the SR-A as displayed in panel e, so its Scatchard plot is not shown.) (e) Gamma counts of total and specific binding of SDIO–DO3A nanoparticles of different degrees of sulfation (at 1 mM [Fe]) to scavenger class A receptors. The more highly sulfated nanoparticles label cells more effectively. (f) Competitive inhibition study of binding to SR-A for SDIO–DO3A-1 using competitors mal-BSA or dextran sulfate. A single SDIO–DO3A-1 concentration (100 μM [Fe] or 22 nM [SDIO]) was used for incubation, while various concentrations of competitor covered 7 orders of magnitude in molar ratio. Error bars represent SEM (n = 3). Full inhibition can be achieved with dextran sulfate, and only partial inhibition, by mal-BSA, suggesting that the binding site for dextran sulfate and the nanoparticles might partially overlap with that for mal-BSA.
