FIGURE 2.
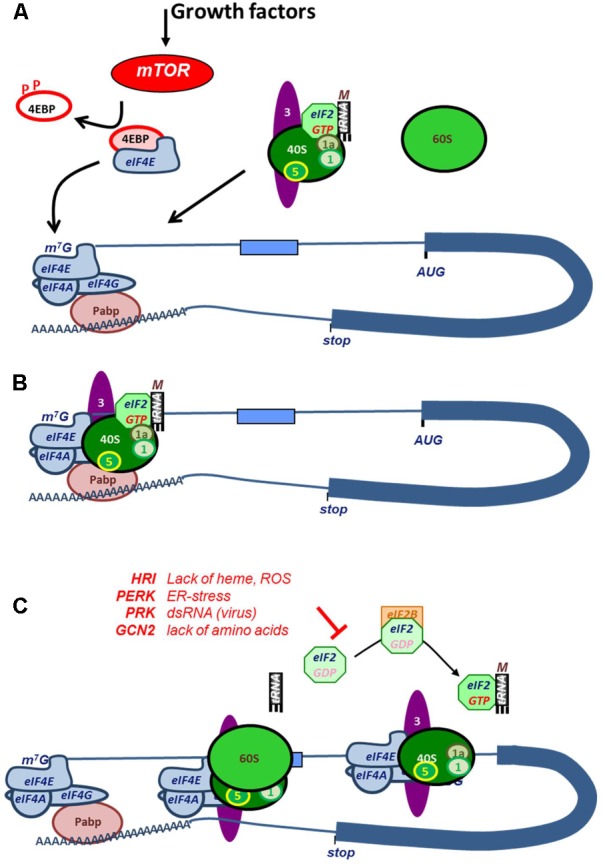
Mechanism and regulation of cap-dependent translation initiation. (A) Growth factor signaling activates phosphorylation of 4EBP by mTOR, which releases the cap-binding factor eIF4E. Upon binding to the mRNA cap, eIF4E associates with helicase eIF4A and scaffold protein eIF4G to form the eIF4F complex. The interaction between the poly(A)-tail binding protein PABP with eIF4G forms a closed loop between cap and tail. Concurrently, the ternary complex, existing of eIF2:GTP and tRNAimet, associates with the 40S ribosomal subunit and eIF1, -1A, -3, and -5 (labeled numerically in the figure). This assembly is referred to as that 43S pre-initiation complex. (B) eIF3 interacts with eIF4G in the eIF4F complex. After docking with the ribosome, the 48S pre-initiation scanning complex is formed, and the transcript is scanned until encountering a start codon, upon which eIF2-bound GTP is hydrolyzed and the 60S ribosomal subunit is recruited. At this stage, initiation of translation is complete, and translational elongation begins. (C) Cap-dependent translation is regulated by the phosphorylation of eIF2. After a round of translation initiation, eIF2 must be recharged with GTP by eIF2B in order for the ternary complex to be reformed and for the 60S ribosomal subunit to be recruited in subsequent cycles of translational initiation. Under stress conditions, eIF2 is phosphorylated by, for example, HRI (heme regulated inhibitor, during heme scarcity), PKR (Protein kinase R, upon recognition of viral dsRNA), PERK (PRK-like endoplasmic reticulum kinase, upon aggregation of malfolded proteins), or GCN (General control non-derepressible, during amino acid starvation), preventing eIF2B from exchanging eIF2-bound GDP for GTP. The lack of unphosphorylated eIF2 leads to translational arrest.
