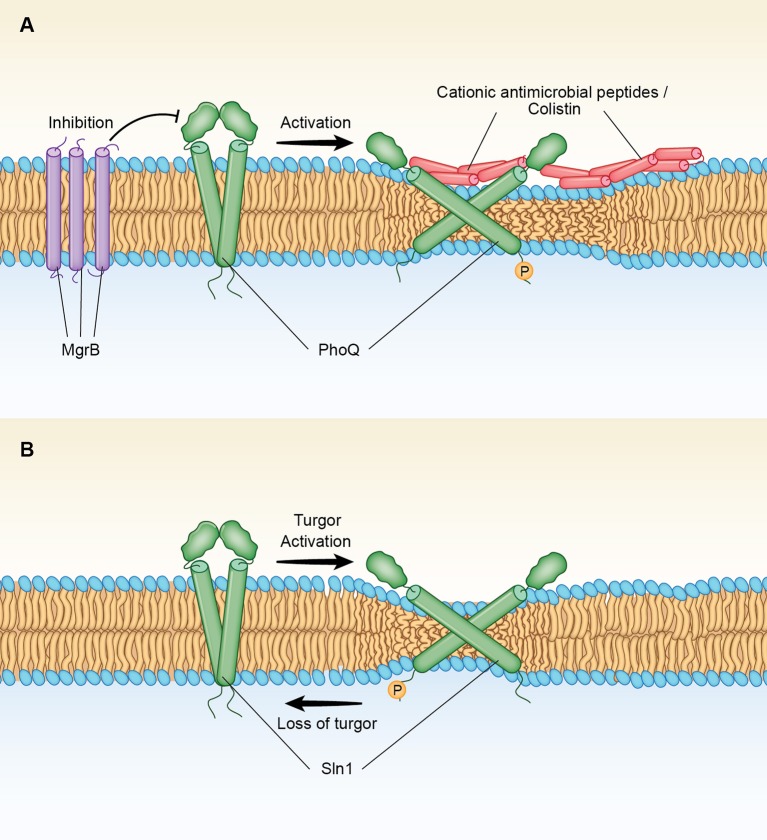
Figure 1.
(A) Mechanism of activation of the two-component PhoQ/PhoP signaling system by AMPs and colistin. Antimicrobial peptides (AMPs) produce a membrane thinning effect that leads to changes in tilting and displacements of the TMs of PhoQ, a bacterial histidine kinase (HK). Such changes convert the HK phosphatase stage, in which the kinase is deactivated, into a phosphorylated state in which the kinase is activated. MgrB is a small transmembrane protein that is part of the PhoQ/PhoP regulatory circuit. MgrB contains a long hydrophobic domain of 20 aminoacids spanning the membrane that upon oxidation of external cysteine residues (see text) may form thicker aggregates that block PhoQ activation. PhoQ activation. Inactivation of mgrB in Gram-negative bacteria results in Colistin resistance through activation of PhoQ/PhoP (see text). (B) Mechanism of activation of HOG by loss of turgor. Sln1 is a histidine kinase (HK) that serves as the osmosensor of the SLN1 branch of the high osmolarity glycerol (HOG) pathway. Increased turgor pressure produces a membrane thinning effect that leads to changes in tilting and displacements of the TMs of Sln1. Such changes convert the phosphorylated state in which the kinase is activated to a phosphatase stage, in which the kinase is deactivated. Accumulation of unphosphorylated Sln1 (and downstream Ssk1) leads to the activation of the HOG pathway.