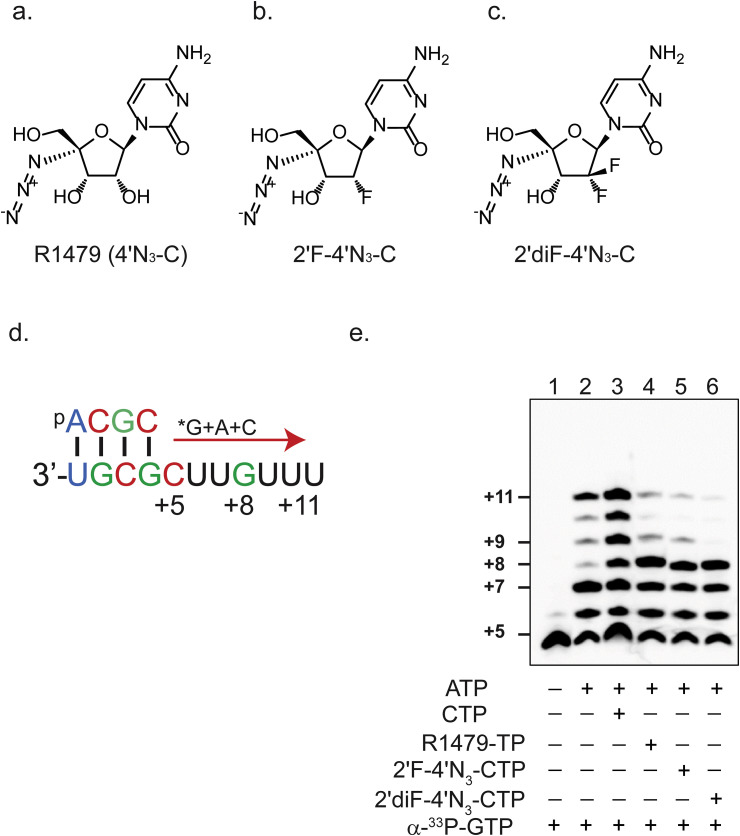
Fig. 1.
Inhibition of RSV polymerase activity by 4′-modified cytidine analogs. Chemical structures of (a) 4′-azidocytidine (R1479), (b) 2′-monofluoro-4′-azidocytidine (2′F-N3-C), and (c) 2′-difluoro-4′-azidocytidine (2′diF-4′N3-C). (d) Schematic of template-directed primer (5′-ACGC) extension assay. *G above the red arrow indicates radioactively labeled guanosine triphosphate (GTP). Similarly, A and C above the red arrow indicate triphosphorylated forms of adenosine (ATP) and cytidine (CTP), respectively. Numbers specify nucleotide positions relative to the first nucleotide of the final primer extension product. (e). Primer extension assay performed with the presence of 33P-radiolabeled GTP alone (lane 1), with ATP (lane 2), and with varying combinations of CTP (lane 3), R1479-TP (lane 4), 2′F-4′N3-CTP (lane 5), or 2′diF-4′N3-CTP (lane 6), as indicated by + and - signs.