Abstract
Gastroduodenal tuberculosis is infrequently seen in day-to-day clinical practice with few cases reported in the literature. It is usually associated with features of gastric outlet obstruction. This is a case series of 4 patients with 2 of them having associated lower gastrointestinal involvement. One of them resembled a growth in the cardia of the stomach which responded to antitubercular drugs. Another had duodenal erosions with portal lymph node enlargement which responded to antitubercular drug treatment. None of the patients required surgical management. Gastroduodenal tuberculosis should be considered with a high degree of suspicion when patients present with gastric outlet obstruction or with endoscopic evidence of ulceronodular disease in areas endemic for tuberculosis.
Keywords: Gastroduodenal, tuberculosis, malignancy, gastric outlet, fistula
Introduction
Tuberculosis (TB) is endemic in India. In the gastrointestinal (GI) tract, ileocecal region is the predominant site of involvement.1 Because tubercular involvement of the gastroduodenal (GD) region is rare, it is underdiagnosed and possibly treated as refractory peptic ulcer disease.2 Involvement of stomach and duodenum is seen in approximately 1% to 2% of cases in various series of abdominal TB.3 There are no specific clinical, radiological, and endoscopic features to diagnose GD TB which poses a great challenge for the clinician. We are reporting a series of 4 cases of GD TB in immunocompetent patients, treated successfully with antitubercular therapy without the need for surgery thus emphasizing need of high suspicion and early initiation of antitubercular drug therapy.
Case Report
Case 1
A 21-year-old man presented with upper abdominal pain since 3 months associated with intermittent vomiting, melena, and 7 kg weight loss. Clinical examination revealed pallor with lump in epigastric region approximately 2 cm ×1.5 cm in size. The lump was nontender, moving with respiration, with normal overlying skin and temperature. Routine blood investigations are summarized in Table 1. There was microcytic hypochromic anemia. Upper GI endoscopy revealed a large deep excavated ulcer in the esophagus at about 25 cm from the incisors and associated ulcer of size 2 cm×1.5 cm with a suspicious fistulous opening seen in the antropyloric region. Computed tomography (CT) of abdomen revealed diffuse circumferential thickening involving the antrum and pylorus together with thickening involving the proximal transverse colon for a length 7 cm, with multiple necrotic perigastric and periportal lymph nodes. Colonoscopy showed nodular mucosa with superficial ulceration in proximal transverse colon. Histopathology from esophageal, antral, and transverse colon ulcer biopsies showed prominent lymphoid aggregate with occasional caseating granuloma and Langerhans giant cell (Figure 2A). Tuberculosis culture using Mycobacteria Growth Indicator Tube (MGIT) was positive from the antral and transverse colon biopsy specimens. Patient was started on antituberculous treatment regimen consisting of isoniazid 5 mg/kg, rifampicin 10 mg/kg, ethambutol 15 mg/kg, and pyrazinamide 25 mg/kg body weight for initial 2 months followed by isoniazid and rifampicin in same dose for another 7 months. Repeat upper GI endoscopy after 4 months of treatment showed healed esophageal and gastric ulcer with healed fistula (Figure 3A). He is asymptomatic and under regular follow-up.
Table 1.
Lab parameters of cases on admission.
| Lab parameters on admission | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Hemoglobin, g% | 5.2 | 9.5 | 11.5 | 8 |
| White blood cells, /mm3 | 4500 | 6000 | 4700 | 8000 |
| Platelet, /mm3 | 240 000 | 198 000 | 234 000 | 265 000 |
| T bilirubin, mg/dL | 0.7 | 0.6 | 0.5 | 0.9 |
| AST, IU | 23 | 33 | 42 | 35 |
| ALT, IU | 32 | 28 | 34 | 32 |
| Creatinine | 0.7 | 0.8 | 0.7 | 1.0 |
| ESR, at end of 1 h | 22 | 54 | 50 | 65 |
| Total protein, mg/dL | 6.4 | 6.2 | 7.2 | 5.2 |
| Albumin, g/dL | 3.5 | 2.7 | 4.1 | 2.4 |
| Mantoux, mm | 18 | 5 | 23 | 16 |
| HIV | Negative | Negative | Negative | Negative |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; HIV, human immune deficiency virus.
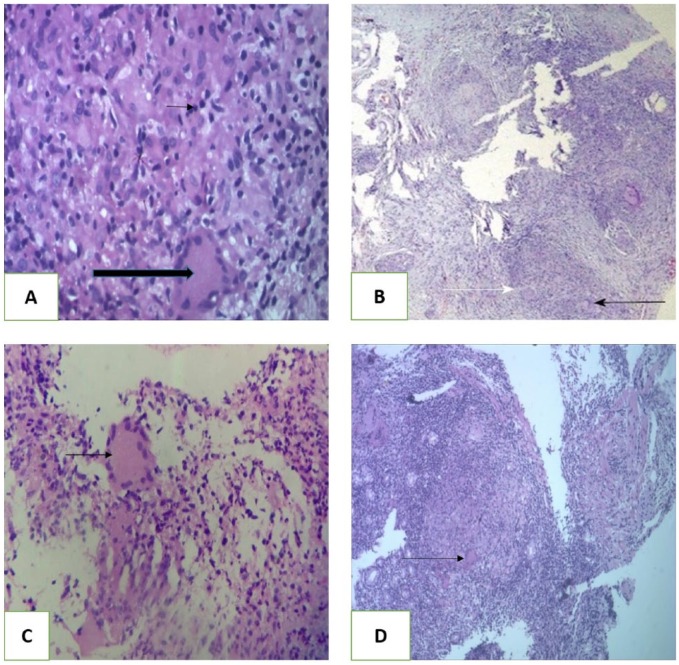
Figure 2.
Histopathologic section (hematoxylin-eosin, original magnification ×100). (A) Case 1—gastric ulcer edge biopsy showing caseating granuloma (large arrow) and Langerhans giant cell (small arrow). (B) Case 2—duodenal erosion biopsy showing granuloma formation (black arrow). White arrow showing Langerhans cell. (C) Case 3—ill-formed Langerhans giant cells with granuloma formation. (D) Case 4—stomach cardia growth biopsy showing caseating granuloma and Langerhans giant cell.
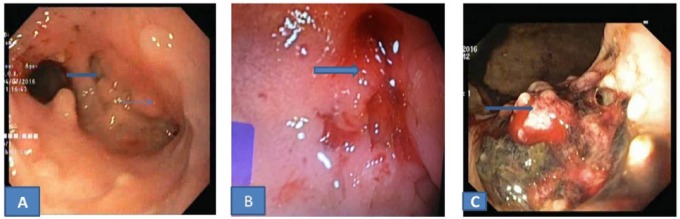
Figure 3.
Upper gastrointestinal endoscopy images after taking anti tubercular therapy. (A) Healed gastric ulcer in case 1 after 4 months of AKT. (B) Healed nodularity with ulceration at D1 and D2 junction in case 2 after 6 months of AKT. (C) Healed ulcer proliferative growth and sinus in case 4 after 9 months of AKT.
Abbreviation: AKT, anti kochs treatment.
Case 2
A 24-year-old woman presented with nonbilious, nonprojectile vomiting, 1 to 2 hours after meals, with low-grade fever for 1 month. There was loss of appetite with significant loss of weight. Clinical examination showed pallor. Routine investigations are summarized in Table 1. There was microcytic hypochromic anemia. Upper GI endoscopy revealed nodularity with ulceration at the junction of the first and second parts of duodenum with a suspicious fistulous opening (Figure 1A). Asymmetric mucosal thickening involving the junction of first and second parts of duodenum with multiple necrotic perigastric and retroperitoneal lymph nodes was noted on CT abdomen. There was also evidence of a suspicious fistulous communication between proximal transverse colon and jejunum along with clumping of bowel loops on contrast-enhanced CT (CECT). Colonoscopy showed long segment ulceronodular lesion in the transverse colon. Biopsy from the duodenal and colonic lesions showed lymphoid aggregate with multiple noncaseating granuloma (Figure 2B). The TB MGIT culture from duodenal biopsy showed growth of Mycobacterium tuberculosis. Patient was given antitubercular drugs for 9 months. She had complete resolution of clinical symptoms with weight gain. Repeat endoscopy after 6 months of antitubercular therapy showed decreased nodularity with healed fistulous opening in the duodenum (Figure 3B).
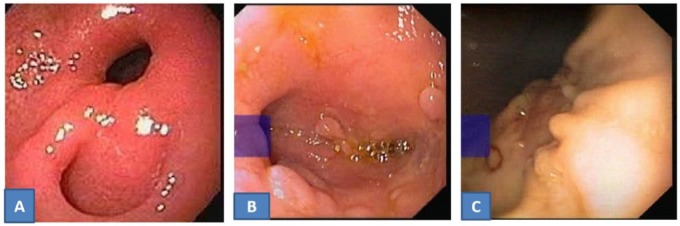
Figure 1.
Upper gastrointestinal endoscopy on admission. (A) Nodularity with ulceration at D1 and D2 junction in case 2. (B) Erosions in duodenum (D1) in case 3. (C) Ulcer proliferative growth with sinus formation in cardia of stomach in case 4.
Case 3
A 22-year-old woman presented with history of upper abdominal pain, mild in intensity, dull aching, nonradiating, associated with occasional nonbilious vomiting since 1 month. There was no clinical evidence of abdominal lump or lymphadenopathy. Routine investigations are summarized in Table 1. There was mild microcytic hypochromic anemia. Upper GI endoscopy revealed multiple small nodules with erosions in the duodenum (Figure 1B). Multiple conglomerated lymph nodes at porta hepatis together with thickening of antropyloric region were seen on CECT. Biopsy from duodenal erosions showed mixed inflammation with multiple noncaseating granulomas (Figure 2C). Duodenal biopsies were positive for TB GeneXpert and TB MGIT culture. Patient has completed 9 months of antitubercular drug therapy. Repeat upper GI endoscopy at the end of 5 months revealed healed duodenal lesions.
Case 4
A 34-year-old woman presented with recurrent vomiting, immediately after food associated with mild, dull aching upper abdominal pain for 3 months. She had anorexia and loss of weight. Routine investigations showed microcytic hypochromic anemia and hypoproteinemia. There was thickening of the gastric fundus and body, multiple necrotic mesenteric lymph nodes, and mild ascites on CECT of the abdomen. Ascitic fluid routine examination showed 200 cells (all lymphocytes) and protein 1.6 g%. Left-sided pleural effusion and multiple hilar necrotic lymph nodes were seen on high-resolution computerized CT of thorax. Pleural fluid had 230 cells (96% lymphocytes). Ascitic and pleural fluid adenosine deaminase were normal (21 and 5 IU/L). An ulceronodular friable lesion (highly suspicious of malignancy) in the gastric cardia with a suspicious adjacent opening suggestive of a sinus tract or a fistula was seen on upper GI endoscopy (Figure 1C). Biopsy of gastric lesion showed caseating epitheloid cell granuloma with Langerhans giant cell in lamina propria (Figure 2D). Patient has completed antitubercular drugs for 9 months and is asymptomatic. Repeat endoscopy at 9 months showed complete healing of the growth-like lesion (Figure 3C).
Discussion
The most common site for GI TB is the ileocecal region, followed by the ascending colon, jejunum, appendix, duodenum, stomach, sigmoid colon, and rectum.1 Probable causes for GD sparing include high acidity, a paucity of lymphoid tissue, and rapid transit of food in the stomach. Long-term therapy with H2 blockers increases the incidence of GD TB; however, none of our patient had given similar history.4 With the extensive use of proton pump inhibitors this incidence may rise.
In about 60% to 70% of patients with GD involvement, there is evidence of TB elsewhere. Two of our patients had colonic involvement and 1 had possible extrapulmonary involvement (pleural effusion). The routes of infection are direct inoculation of the mucosa, hematogenous spread, or extension from neighboring tuberculous lesion.5
Gastric TB with unusual presentation a case as reported by Okoro and Komolafe was an elderly man suspected to have abdominal malignancy but subsequently found to be extensive, complicated gastric TB coexisting with chronic peptic ulcer disease.6 We had similar patient who had growth in cardia mimicking malignancy which resolved completely with antitubercular drugs. The clinical presentation of GD TB includes a wide range of symptoms such as epigastric pain, vomiting, weight loss, hematemesis, perforations, gastric outlet obstruction, and surgical obstructive jaundice.7 The cause of GD obstruction can be either stricture or external compression by lymph node.8 Fistulous communication can occur between the duodenum and the bile duct, colon, or renal pelvis.9 In our case series, 1 patient had fistulous opening in stomach with communication with transverse colon and also another patient, young woman, with suspicious fistulous tract between transverse colon and jejunum. Both the patients responded well to treatment with successful closure of fistulous opening with antitubercular therapy alone. Chest imaging may show evidence of pulmonary TB in up to 20% of cases.10 Thickening of the gastric or duodenal wall, associated with enlarged local lymph nodes, is often visible on CT scan and may be the only clue to the diagnosis as our patient had similar portal lymph node with duodenal erosion. Upper GI endoscopy may reveal wide variety of findings including nodularity, ulcerations, thickening, erythema, fistulous opening, and deformity involving the antropyloric region and duodenum.11 It may also present as multiple shallow ulcers, especially on the lesser curvature of the stomach or as a hypertrophic submucosal mass.12 Even in ulcerated lesions, endoscopic biopsy rarely reveals granulomas because of the predominantly submucosal location of these lesions. In a review of 27 patients who underwent endoscopic biopsies of duodenal TB, 20 had nonspecific duodenitis and granulomas were found in only 7. Acid-fast bacilli (AFB) are rarely recovered from the biopsy material, although fine-needle aspiration cytology may have a higher yield.13 However, in recent studies, granulomatous inflammation was demonstrated in 90% to 100% of patients using a combination of multiple endoscopic biopsies and endoscopic mucosal resection, although AFB were rarely demonstrated.14,15
In a minority of cases, it is possible to isolate mycobacteria in culture from gastric lavage. To contrary with previous reports all 4 patients from our case series had granuloma and 3 patients had positive culture. When diagnosis of TB is established, most lesions regress with appropriate antitubercular treatment and do not require excision.16 Even in patients with strictures, endoscopic balloon dilatation has been successful.17 Elective surgery should be required only for complications such as obstruction, fistula formation, or intractable ulceration.
In conclusion, a high level of suspicion is required for the diagnosis of GD TB, especially in endemic countries such as India, even in patient having growth in cardia of stomach. Once identified correctly, these patients can have good outcome with timely administration of antitubercular therapy and only few require endoscopic or surgical interventions in contrast to the previous case reports.
Footnotes
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions: SU wrote the first draft of the manuscript, contributed to the writing of the manuscript, agreed with manuscript results and conclusions, jointly developed the structure and arguments for the paper, made critical revisions, and approved the final version. SU, RS, VZ, SC,QC and PR analyzed the data. All authors reviewed and approved the final manuscript.
ORCID iD: Suhas Udgirkar  https://orcid.org/0000-0002-1275-4833
https://orcid.org/0000-0002-1275-4833
References
- 1. Gorbach S. Tuberculosis of the gastrointestinal tract. In: Sleisenger M, Fordtran J.eds. Gastrointestinal Diseases. Vol 2, 4th ed. Philadelphia, PA: W.B. Saunders; 1989:363–722. [Google Scholar]
- 2. Mukherji B, Singhal AK. Tuberculosis of the stomach and the stomach in tuberculosis: a review with particular reference to gross pathology and gastroscopic diagnosis. Am Rev Tuberc. 1950;61:1163–1130. [DOI] [PubMed] [Google Scholar]
- 3. Tandon HD, Prakash A. Pathology of intestinal tuberculosis and its distinction from Crohn’s disease. Gut. 1972;13:260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marshall JB. Tuberculosis of the gastrointestinal tract and peritoneum. Am J Gastroenterol. 1993;88:989–999. [PubMed] [Google Scholar]
- 5. Gupta B, Mathew S, Bhalla S. Pyloric obstruction due to gastric tuberculosis—an endoscopic diagnosis. Postgrad Med J. 1990;66:63–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Okoro EO, Komolafe OF. Gastric tuberculosis: unusual presentations in two patients. Clin Radiol. 1999;54:257–259. [DOI] [PubMed] [Google Scholar]
- 7. Sharma BC, Prasad H, Bhasin DK, Singh K. Gastroduodenal tuberculosis presenting with massive hematemesis in a pregnant woman. J Clin Gastroenterol. 2000;30:336. [DOI] [PubMed] [Google Scholar]
- 8. Woudstra M, van Tilburg AJ, Tjen JS. Two young Somalians with gastric outlet obstruction as a first manifestation of gastroduodenal tuberculosis. Eur J Gastroenterol Hepatol. 1997;9:393–395. [DOI] [PubMed] [Google Scholar]
- 9. Chaudhary A, Bhan A, Malik N, Dilawari JB, Khanna SK. Choledocho-duodenal fistula due to tuberculosis. Indian J Gastroenterol. 1989;8:293–294. [PubMed] [Google Scholar]
- 10. Mukherji B, Singhal AK. Intestinal tuberculosis. Proc Assoc Surg East Afr. 1968;2:71–75. [Google Scholar]
- 11. Tandon RK, Pastakia B. Duodenal tuberculosis as seen by duodenoscopy. Am J Gastroenterol. 1976;66:483–486. [PubMed] [Google Scholar]
- 12. Rohwedder JJ. Abdominal tuberculosis: a disease poised for reappearance. N Y State J Med. 1989;89:252–254. [PubMed] [Google Scholar]
- 13. Gilinsky NH, Marks IN, Kottler RE, Price SK. Abdominal tuberculosis. A 10-year review. S Afr Med J. 1983;64:849–857. [PubMed] [Google Scholar]
- 14. Puri AS, Sachdeva S, Mittal VV. Endoscopic diagnosis, management and outcome of gastroduodenal tuberculosis. Indian J Gastroenterol. 2012;31:125–129. [DOI] [PubMed] [Google Scholar]
- 15. De A, Lamoria S, Dhawan S. Duodenal tuberculosis: dig deep to diagnose. Trop Doct. 2016;46:172–174. [DOI] [PubMed] [Google Scholar]
- 16. Anand BS, Nanda R, Sachdev GK. Response of tuberculous stricture to antituberculous treatment. Gut. 1988;129:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vij JC, Ramesh GN, Choudhary V, Malhotra V. Endoscopic balloon dilation of tuberculous duodenal strictures. Gastrointest Endosc. 1992;38:510–511. [DOI] [PubMed] [Google Scholar]