Abstract
Chronic thromboembolic pulmonary hypertension (CTEPH) is a complication of unresolved organised pulmonary emboli/thrombi obstructing the major pulmonary arteries. The aim of this study was to estimate the incidence and risk factors of CTEPH in a cohort with first venous thromboembolism (VTE). This was a population-based cohort study of patients with first VTE and no active cancer in England between 2001 and 2012. CTEPH was assessed using a rigorous case-ascertainment algorithm. Risk factors for CTEPH were studied using a nested case-control approach by matching CTEPH cases to VTE patients without CTEPH. Adjusted odds ratios (OR) of comorbidities were estimated from conditional logistic regression. During 81,413 person-years of follow-up among 23,329 patients with first VTE (mean follow-up 3.5 years; maximum 11.0 years) 283 patients were diagnosed with CTEPH (incidence rate 3.5 per 1000 person-years); cumulative incidence was 1.3% and 3.3% at 2 and 10 years after pulmonary embolism, and 0.3% and 1.3% following deep vein thrombosis (DVT), respectively. Risk factors for CTEPH included age over 70, OR 2.04 (95% CI 1.23 to 3.38), female gender, 1.44 (1.06 to 1.94), pulmonary embolism at first VTE, 3.11 (2.23 to 4.35), subsequent pulmonary embolism and DVT, 3.17 (2.02 to 4.96) and 2.46 (1.34 to 4.51) respectively, chronic obstructive pulmonary disease 3.17 (2.13 to 4.73), heart failure 2.52 (1.76 to 3.63) and atrial fibrillation, 2.42 (1.71 to 3.42). CTEPH develops most commonly after pulmonary embolism and less frequently after DVT. Awareness of risk factors may increase referrals to specialised centres for confirmation of CTEPH and initiation of specific treatment.
Keywords: pulmonary hypertension, incidence, predictive factors, deep vein thrombosis, pulmonary embolism
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a life-threatening condition caused by unresolved and organised pulmonary emboli/thrombi obstructing the major pulmonary arteries. The absence of thrombus resolution causes chronic obstruction with increased pulmonary vascular resistance, progressive pulmonary hypertension and right heart failure. Diagnostic criteria of CTEPH are a sustained elevation of mean pulmonary arterial pressure greater than 25 mmHg at rest that persists at least 3 months after effective anticoagulation for pulmonary embolism, and when one or more mismatched segmental or larger perfusion defects are detected by ventilation-perfusion lung scanning.1,2 Symptoms are usually non-specific and made worse by exertion. They are related to progressive right ventricular dysfunction and include dyspnoea, oedema, fatigue, weakness, angina and syncope, and less commonly haemoptysis.3,4
The true incidence of CTEPH is unknown. Classical estimates of the frequency of CTEPH, based on small samples with a mean follow-up of up to 3.5 years, refer to the number of CTEPH cases per survived pulmonary thromboembolic events, and the reported cumulative incidences range between 0.4% and 9.1% of pulmonary thromboembolic events.5–12 Incidence estimates vary and mainly depend on the inclusion criteria of the populations studied (most studies restricted to patients with acute pulmonary embolism), the duration of observation and whether the diagnostic process of CTEPH was triggered by clinical symptoms or routine screening. As 30% of patients with CTEPH have no history of venous thromboembolism (VTE), CTEPH may be undiagnosed and optimal treatment delayed.13 CTEPH is associated with high mortality, particularly in the presence of serious comorbidities.6,14
Few studies have investigated risk factors for CTEPH, and population studies on the incidence, complications and risk factors of CTEPH are limited.13,15,16 The objective of the present study was to estimate the incidence and mortality of CTEPH in a cohort with first (VTE) and to explore risk factors for CTEPH.
Methods
Design, study setting and participants
A population-based cohort study in patients with first VTE was conducted using data obtained from the subset of the UK Clinical Practice Research Datalink (CPRD), linked to the Hospital Episodes Statistics (HES) and to the Office for National Statistics mortality data. CPRD comprises data from ∼8% of the UK population and linkage to HES is available for ∼6% of the English population. CPRD is based on information documented in the primary care setting and includes demographics, medical history, symptoms and diagnoses recorded with Read medical codes, unstructured medical notes, letters to and from secondary care and prescriptions issued by the general practitioner (GP). HES include dates of hospital admission and discharge, primary and other main reasons for treatment recorded with ICD-10 codes, and surgical operations and procedures performed during hospital stay recorded with OPCS-4 codes. Office for National Statistics mortality data contain the date and cause of death recorded as ICD-10 in death certificates.
Study population
The study cohort of incident non-fatal first VTE was formed based on a previously described and validated algorithm.17 Briefly, we identified all patients diagnosed for the first time with VTE between 1 January 2001 and 31 March 2012 and excluded patients with less than 1 year of medical history before cohort entry. In contrast to the previously published VTE cohort, the study period was extended until March 2012 and the study cohort was restricted to patients aged <85 years, to patients without active cancer at their first VTE (defined as recording for cancer or chemotherapy ± 90 days to first VTE) and to patients with at least 90 days of observation following the first VTE. The date of the first VTE defined the start of observation. Patients were followed until a recording of CTEPH, death, end of registration with the practice, start of active cancer episode or end of the study period (31 March 2012), whichever came first.
Definition of CTEPH
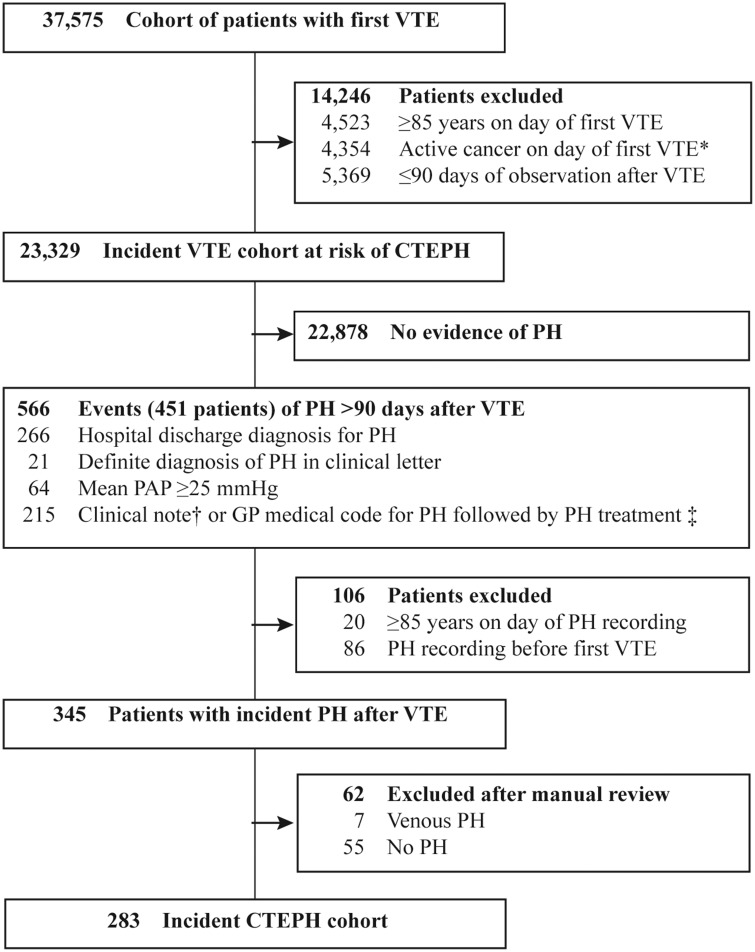
The primary study outcome was CTEPH. A case-ascertainment algorithm was developed based on the guidelines for the diagnosis and treatment of pulmonary hypertension (PH)18–20 and expert clinical input, and then modified by an iterative approach following the manual review of case summaries. The CTEPH ascertainment algorithm included evidence of increased pulmonary artery pressure recorded at least 90 days after the initial deep vein thrombosis (DVT) or pulmonary embolism from three sources: (a) hospital-based information consisted of discharge diagnoses of PH, (b) GP-based information consisted of GP-based diagnoses of increased pulmonary artery pressure and PH-specific treatment and (c) anonymised electronic clinical notes with information of hospital and specialist letters and of mean pulmonary artery pressure of ≥ 25 mmHg; see Fig. 1 and online supplement for details.
Figure 1.
Ascertainment of patients with first CTEPH >90 days after VTE.
CTEPH: Chronic thromboembolic pulmonary hypertension; GP: General practitioner; PAP: Pulmonary artery pressure; PH: Pulmonary hypertension; VTE: Venous thromboembolism.
*: Defined as recording for cancer or chemotherapy ±90 days to first VTE; †: Definite free text note for PH diagnosis not recorded in a clinical discharge letter or free text note for suspicion of PH or “mild” PH; ‡: Treatment for PH defined as one of the following events: (a) Prescription or clinical note for specific PH medication, (b) prescription for unspecific PH medication without history of erectile dysfunction, or (c) OPCS procedure code or clinical note for pulmonary endarterectomy or a transplantation of the lung.
Case summaries consisting of diagnostic procedures, symptoms, signs, diagnoses from primary and secondary care, in-hospital procedures and PH-specific medications, were reviewed by a clinical expert (A.P.), who was the final arbiter as to whether a potential case was “no CTEPH”, “possible”, “probable” or “definite CTEPH”. The day of onset of CTEPH was the day when it became clear that the patient had chronic PH following a venous thromboembolism. Potential CTEPH events were excluded if there was a previous recording of any PH before the first episode of VTE, if there was no evidence of PH, if the clinical notes stated pulmonary venous hypertension, or if the PH was acute and resolved within 90 days of active anticoagulant therapy. Cases of PH were assessed as “definite CTEPH” if a pulmonary endarterectomy or lung transplantation was performed or deemed to have operable CTEPH; “probable CTEPH” if CTEPH was confirmed in an anonymised clinical letter, or when the hospital or GP-based diagnosis of PH was followed by use of prostacyclin, endothelin receptor antagonists, or of phosphodiesterase-5 inhibitors in the absence of a recording for erectile dysfunction. All remaining cases were assessed as “possible CTEPH”.
Case-control selection
A nested case-control analysis among patients with first VTE was conducted to explore risk factors for CTEPH. For this purpose, a reference group per case of CTEPH was generated by randomly selecting up to 10 controls per case after matching on GP practice, calendar day of first VTE ± 1 year, and time between first VTE and index day (day of CTEPH).
Covariates
Covariates for the risk factor analysis included factors shown to be associated with CTEPH, including age, gender, body mass index (BMI), smoking status, type of first VTE (DVT or pulmonary embolism), source of diagnosis of first VTE (GP, clinical letter, hospital discharge) and recurrent VTE, and furthermore, various comorbidities, that is, asthma, chronic obstructive pulmonary disease (COPD), diabetes, heart failure (HF), hypothyroidism, inflammatory bowel disease, systemic sclerosis and systemic lupus erythematosus, defined from any hospital discharge or GP-based diagnoses, and atrial fibrillation (AF) defined with additional in-hospital procedures.3,11,13 Splenectomy was defined from in-hospital procedures or GP recordings. Anticoagulation was identified using GP-issued prescriptions for oral anticoagulants, parenteral anticoagulants in therapeutic dose, and INR test results. All code sets were developed in collaboration with the clinical experts.
Data analysis
Crude age- and gender-specific incidence rates of CTEPH and incidence rates of CTEPH by time since first VTE were provided from the number of incident CTEPH cases divided by the cumulative person-years of the respective stratum in the VTE cohort.
Competing risk survival analyses accounted for mortality, cancer and recurrent pulmonary embolism as competing risks for the overall cumulative incidence of CTEPH following VTE and accounted for mortality and cancer for the cumulative incidence of CTEPH in the subset of patients with a recurrent pulmonary embolism.
All-cause mortality was estimated for all cases of CTEPH and compared with up to 10 patients with VTE, but without CTEPH, matched on gender, age, calendar day of first VTE ± 1 year, and time between first VTE and CTEPH. Competing risk survival analyses were conducted to estimate the crude all-cause mortality risk in those with CTEPH and the age- and gender-matched cohort, accounting for cancer as competing risk.
A case-control analysis was performed to estimate adjusted odds ratios (ORs) from conditional logistic regression models using all covariates as independent variables and CTEPH as the dependent variable. The covariate anticoagulation use was defined as any use of anticoagulants after the first VTE. While the main case-control analysis used controls matched from the GP practice, sensitivity analyses were conducted using age and gender-matched controls, by stratifying the main results on VTE type (DVT and pulmonary embolism), and by bringing the index day (day of CTEPH) 90 days forward to account for delays of the CTEPH diagnosis.
All statistical procedures were performed using the Stata MP Version 14.1 (StataCorp LP). The study protocol was approved by the Independent Scientific Advisory Committee (ISAC) for CPRD research, ISAC protocol 14_028MnAR.
Results
The incident VTE cohort consisted of 37,575 patients. After excluding patients with an age ≥85 years at first VTE (4523), patients with active cancer at first VTE (4354) and patients with less than 90 days of observation (5369), the incident VTE and at risk of CTEPH cohort comprised 23,329 patients with 81,413 person-years of follow-up (mean follow-up 3.5 years, maximum follow-up 11.0 years), 12,022 (51.5%) with DVT and 11,307 (48.5%) with pulmonary embolism, Fig. 1. The mean age of the VTE cohort was 60.2 years, 59.4 for those with DVT and 61.1 for pulmonary embolism, and 48.9% were male with comparable sex ratios for pulmonary embolism and deep vein thrombosis, Table 1.
Table 1.
Demographics and clinical characteristics of VTE cohort at risk for CTEPH*
| DVT N = 12,022 | PE N = 11,307 | VTE N = 23,329 | |
|---|---|---|---|
| Male sex | 5926 (49.3) | 5488 (48.5) | 11,414 (48.9) |
| Age at VTE diagnosis (years) | 59.4 ± 17.5 | 61.1 ± 16.4 | 60.2 ± 17.0 |
| <40 | 2087 (17.4) | 1464 (12.9) | 3551 (15.2) |
| 40–49 | 1332 (11.1) | 1263 (11.2) | 2595 (11.1) |
| 50–59 | 1824 (15.2) | 1718 (15.2) | 3542 (15.2) |
| 60–69 | 2445 (20.3) | 2559 (22.6) | 5004 (21.4) |
| 70–79 | 2972 (24.7) | 3099 (27.4) | 6071 (26.0) |
| 80–84 | 1362 (11.3) | 1204 (10.6) | 2566 (11.0) |
| Body mass index† (kg/m2) | 28.2 ± 6.2 | 28.7 ± 6.5 | 28.4 ± 6.3 |
| <18.5 | 216 (2.1) | 181 (1.8) | 397 (2.0) |
| 18.5–24.9 | 3096 (30.6) | 2638 (26.8) | 5734 (28.7) |
| 25.0–29.9 | 3545 (35.0) | 3687 (37.5) | 7232 (36.2) |
| 30.0–34.9 | 2002 (19.8) | 1955 (19.9) | 3957 (19.8) |
| ≥35.0 | 1261 (12.5) | 1376 (14.0) | 2637 (13.2) |
| Unknown BMI | 1902 (15.8) | 1470 (13.0) | 3372 (14.5) |
| Alcohol consumption† | |||
| Never | 1888 (18.9) | 1849 (19.1) | 3737 (19.0) |
| Ex drinker | 304 (3.0) | 305 (3.2) | 609 (3.1) |
| Current drinker | 7805 (78.1) | 7504 (77.7) | 15,309 (77.9) |
| Alcohol intake unknown | 2025 (16.8) | 1649 (14.6) | 3674 (15.7) |
| Smoking status† | |||
| Never | 5640 (50.2) | 5426 (50.2) | 11,066 (50.2) |
| Ex smoker | 3002 (26.7) | 3271 (30.3) | 6273 (28.5) |
| Current smoker | 2585 (23.0) | 2105 (19.5) | 4690 (21.3) |
| Smoking status unknown | 795 (6.6) | 505 (4.5) | 1300 (5.6) |
BMI: Body mass index (weight in kilograms divided by the square of the height in metres); CTEPH: Chronic thromboembolic pulmonary hypertension; DVT: Deep vein thrombosis; PE: Pulmonary embolism; SD: Standard deviation; VTE: Venous thromboembolism.
: Plus–minus values are means ± SD and all others are n (%); †: Last information available before VTE diagnosis.
The CTEPH algorithm identified 345 potential cases of incident PH more than 90 days after the first VTE (Fig. 1). Patient summaries of all potential cases with any clinical notes indicative of PH were provided to a clinical expert in PH (A.P.) for review and assessment. The clinical expert confirmed the CTEPH diagnosis in 283 patients; 200 were assessed as possible, 83 as probable or definite, and 62 were excluded, Fig. 1. The sensitivity of our CTEPH ascertainment algorithm without the manual review of the clinical expert was estimated at 85.8%, and the specificity at 99.2% (see online supplement for details on methodology of sensitivity and specificity calculation). The specificity of the algorithm including the manual review of clinical notes was 100%, as all false positives were excluded from the CTEPH cohort.
The overall incidence rate of CTEPH in VTE patients was 3.5 (95% confidence interval (CI): 3.1–3.9) per 1000 person-years, and similar in males and females. The age-specific incidence rate increased with age from 1.7 (95% CI 1.0–2.8) in patients 40–49 years of age to 5.7 (95% CI 4.3–7.4) in patients 80–84 years of age (Table 2 and supplementary Figure 1). Of all CTEPH cases following a first DVT, 42.9% were diagnosed within 2 years in contrast to 62.0% following an initial pulmonary embolism (Table 3). When stratified by type of VTE, the incidence rate was 1.6 (1.3–2.0) per 1000 person-years following a DVT and 5.6 (4.9–6.4) following a pulmonary embolism. When stratified by time since VTE, the incidence rate of CTEPH peaked in the 91 to 182 days following the VTE 8.5 (95% CI 6.3–11.3) per 1000 person-years and then declined to 2.5 (2.1–3.0) after more than 2 years (Table 3).
Table 2.
Crude age- and gender-specific incidence rates of CTEPH after acute VTE
| Male |
Female |
Total |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Cases of CTEPH | Person- years | Incidence rate (95% CI)* | Cases of CTEPH | Person- years | Incidence rate (95% CI)* | Cases of CTEPH | Person- years | Incidence rate (95% CI)* |
| <40 | <5 | 4026 | – | 11 | 6466 | 1.7 (0.8–3.0) | 13 | 10492 | 1.2 (0.7–2.1) |
| 40–49 | 9 | 4924 | 1.8 (0.8–3.5) | 8 | 4945 | 1.6 (0.7–3.2) | 17 | 9869 | 1.7 (1.0–2.8) |
| 50–59 | 19 | 6920 | 2.7 (1.7–4.3) | 10 | 5902 | 1.7 (0.8–3.1) | 29 | 12821 | 2.3 (1.5–3.2) |
| 60–69 | 25 | 9800 | 2.6 (1.7–3.8) | 30 | 8069 | 3.7 (2.5–5.3) | 55 | 17869 | 3.1 (2.3–4.0) |
| 70–79 | 55 | 9776 | 5.6 (4.2–7.3) | 60 | 11,070 | 5.4 (4.1–7.0) | 115 | 20846 | 5.5 (4.6–6.6) |
| 80–84 | 20 | 3807 | 5.3 (3.2–8.1) | 34 | 5708 | 6.0 (4.1–8.3) | 54 | 9515 | 5.7 (4.3–7.4) |
| Total | 130 | 39,253 | 3.3 (2.8–3.9) | 153 | 42,160 | 3.6 (3.1–4.3) | 283 | 81413 | 3.5 (3.1–3.9) |
CTEPH: Chronic thromboembolic pulmonary hypertension; CI: Confidence interval; VTE: Venous thromboembolism.
: Incidence rate per 1000 person-years with 95% CI. Incidence rate for age <40 in males not presented as based on <5 cases of CTEPH.
Table 3.
Crude incidence rate of CTEPH by time since VTE, stratified by type of VTE
| Time after VTE | Cases of CTEPH | Person- years | Incidence rate (95% CI)* |
|---|---|---|---|
| VTE (DVT or PE) | |||
| 91–182 days | 48 | 5657 | 8.5 (6.3–11.3) |
| 183–365 days | 61 | 10,189 | 6.0 (4.6–7.7) |
| 1–2 years | 53 | 17,049 | 3.1 (2.3–4.1) |
| >2 years | 121 | 48,518 | 2.5 (2.1–3.0) |
| Total | 283 | 81,413 | 3.5 (3.1–3.9) |
| DVT | |||
| 91–182 days | 8 | 2922 | 2.7 (1.2–5.4) |
| 183–365 days | 11 | 5302 | 2.1 (1.0–3.7) |
| 1–2 years | 11 | 8920 | 1.2 (0.6–2.2) |
| >2 years | 40 | 26,118 | 1.5 (1.1–2.1) |
| Total | 70 | 43,263 | 1.6 (1.3–2.0) |
| PE | |||
| 91–182 days | 40 | 2735 | 14.6 (10.4–19.9) |
| 183–365 days | 50 | 4887 | 10.2 (7.6–13.5) |
| 1–2 years | 42 | 8129 | 5.2 (3.7–7.0) |
| >2 years | 81 | 22,399 | 3.6 (2.9–4.5) |
| Total | 213 | 38,150 | 5.6 (4.9–6.4) |
CTEPH: Chronic thromboembolic pulmonary hypertension; CI: Confidence interval; DVT: Deep vein thrombosis; PE: Pulmonary embolism; VTE: Venous thromboembolism. *: Incidence rate per 1000 person-years with 95% CI.
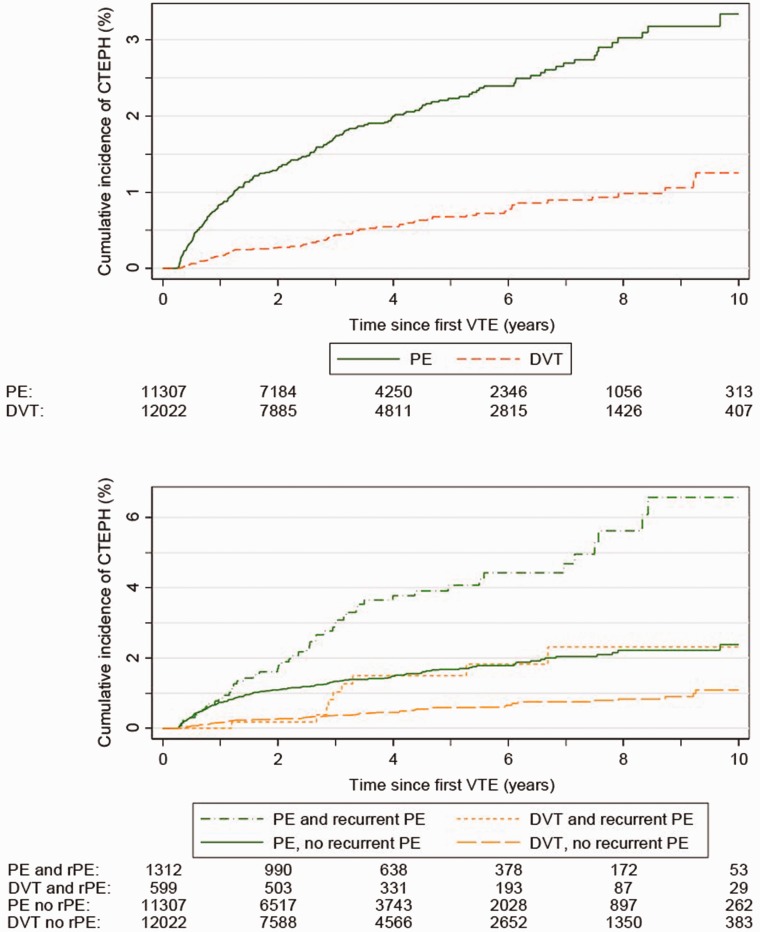
The overall cumulative 10-year incidence of CTEPH was 2.2% following any VTE, 3.3% after a first pulmonary embolism, and 1.3% following a first DVT. Cumulative 10-year CTEPH incidences were higher in the presence of a recurrent pulmonary embolism: 6.6% and 2.3% in those with a new pulmonary embolism following the first pulmonary embolism and DVT respectively (Fig. 2).
Figure 2.
Cumulative incidence of CTEPH by time since first VTE (deep vein thrombosis or pulmonary embolism) overall (upper panel), and by recurrent pulmonary embolism (lower panel).
CTEPH: Chronic thromboembolic pulmonary hypertension; DVT: Deep vein thrombosis; PE: Pulmonary embolism; rPE: recurrent PE; VTE: Venous thromboembolism.
The overall mortality rate in the 10 years following the first CTEPH diagnosis was 14.1 (11.1–17.5) per 100 person-years. Mortality rates peaked in the 90 days following the CTEPH diagnosis, at 32.7 (20.2–49.9) per 100 person-years and then decreased to 17.5 and 11.0 per 100 person-years after 91 to 365 days and 1 to 2 years after the initial VTE respectively (Table 4).
Table 4.
Mortality rate following CTEPH diagnosis
| Time after CTEPH | Deaths | Person- years | Mortality rate (95%-CI)* |
|---|---|---|---|
| 1–90 days | 21 | 64 | 32.7 (20.2–49.9) |
| 91–365 days | 26 | 149 | 17.5 (11.4–25.6) |
| 1–2 years | 14 | 128 | 11.0 (6.0–18.4) |
| >2 years | 18 | 221 | 8.1 (4.8–12.9) |
| Total | 79 | 562 | 14.1 (11.1–17.5) |
CTEPH: Chronic thromboembolic pulmonary hypertension; CI: Confidence interval.
: Crude mortality rate per 100 person-years with 95% CI.
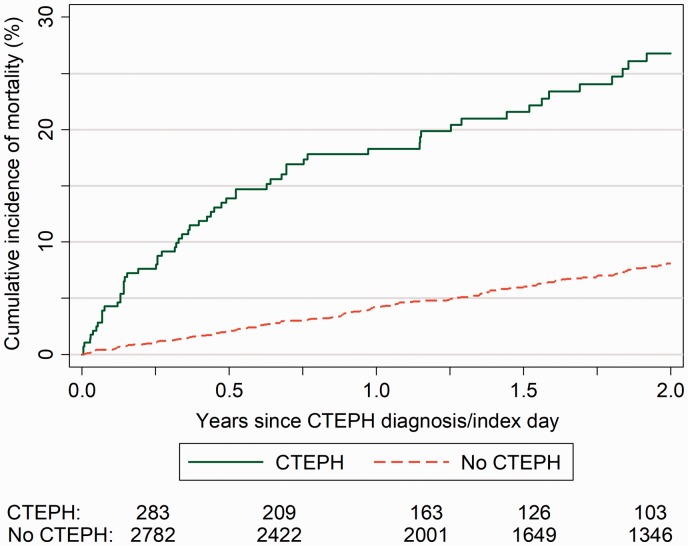
The probability of mortality within 2 years was significantly higher in the CTEPH cohort compared with the control cohort matched on year of birth and gender, and was 26.8% compared with 8.1% in the matched VTE cohort without CTEPH (Fig. 3).
Figure 3.
Survival estimate of mortality from any cause following the cohort of patients with CTEPH and an age and gender-matched reference cohort of VTE patients without CTEPH.
CTEPH: Chronic thromboembolic pulmonary hypertension; VTE: Venous thromboembolism.
The mean age of the 283 CTEPH patients was 68.2 years, and 79.2% were 60 years or older. Of the total, 130 (45.9%) were in men and 153 (54.1%) in women.
The case-control analysis of 283 cases and 2356 GP practice-matched controls showed age over 70 to be associated with CTEPH compared with age below 50, OR 2.04 (1.23–3.38), while women had a borderline increased risk, OR 1.44 (1.06–1.94). Pulmonary embolism at first VTE with or without DVT was a strong risk factor for CTEPH, with an adjusted OR of 3.11 (2.23–4.35). New pulmonary embolisms with or without DVT were also associated with an increased risk of CTEPH, with an OR of 3.17 (2.02–4.96), as was a new DVT only, with OR 2.46 (1.34–4.51) compared with those without a recurrent event (Table 5).
Table 5.
Risk factors for CTEPH
| Risk factor | Cases n (%) | Controls n (%) | Crude OR (95% CI) | Adjusted OR* (95% CI) |
|---|---|---|---|---|
| Total | 283 | 2356 | ||
| Age† (years) | ||||
| 0–49 | 30 (10.6) | 615 (26.1) | 1 | 1 |
| 50–69 | 84 (29.7) | 866 (36.8) | 2.07 (1.34–3.20) | 1.41 (0.86–2.32) |
| 70–.84 | 169 (59.7) | 875 (37.1) | 4.25 (2.82–6.42) | 2.04 (1.23–3.38) |
| Gender | ||||
| Male | 130 (45.9) | 1137 (48.3) | 1 | 1 |
| Female | 153 (54.1) | 1219 (51.7) | 1.09 (0.85–1.40) | 1.44 (1.06–1.94) |
| First VTE | ||||
| DVT | 70 (24.7) | 1231 (52.2) | 1 | 1 |
| PE ± DVT | 213 (75.3) | 1125 (47.8) | 3.65 (2.72–4.91) | 3.11 (2.23–4.35) |
| Recurrent VTE | ||||
| None | 216 (76.3) | 2089 (88.7) | 1 | 1 |
| DVT | 20 (7.1) | 140 (5.9) | 1.37 (0.83–2.26) | 2.46 (1.34–4.51) |
| PE ± DVT | 47 (16.6) | 127 (5.4) | 3.52 (2.42–5.13) | 3.17 (2.02–4.96) |
| Other comorbidities‡ | ||||
| Inflammatory bowel disease | 7 (2.5) | 49 (2.1) | 1.27 (0.57–2.85) | 1.28 (0.51–3.19) |
| Systemic lupus erythematosus | 5 (1.8) | 12 (0.5) | 3.61 (1.26–10.30) | 2.79 (0.81–9.56) |
| Asthma | 55 (19.4) | 412 (17.5) | 1.15 (0.84–1.59) | 0.81 (0.55–1.20) |
| COPD | 82 (29.0) | 192 (8.1) | 4.91 (3.60–6.71) | 3.17 (2.13–4.73) |
| Atrial fibrillation | 113 (39.9) | 291 (12.4) | 4.58 (3.49–6.02) | 2.42 (1.71–3.42) |
| Heart failure | 96 (33.9) | 187 (7.9) | 5.50 (4.10–7.38) | 2.52 (1.76–3.63) |
| Hypothyroidism | 31 (11.0) | 176 (7.5) | 1.48 (0.98–2.24) | 1.08 (0.66–1.78) |
| Splenectomy | 6 (2.1) | 10 (0.4) | 5.65 (2.05–15.59) | 4.30 (1.29–14.35) |
| Diabetes | 55 (19.4) | 288 (12.2) | 1.66 (1.20–2.28) | 1.21 (0.81–1.80) |
CI: Confidence interval; COPD: Chronic obstructive pulmonary disease; CTEPH: Chronic thromboembolic pulmonary hypertension; DVT: Deep vein thrombosis; OR: Odds ratio; PE: Pulmonary embolism; VTE: Venous thromboembolism.
: Derived from multivariate conditional logistic regression model adjusting for all presented covariates, and body mass index, smoking status, source of first VTE diagnosis and anticoagulant use; †: At index day; ‡: Any history before index day.
Of the comorbidities studied, splenectomy increased the risk of CTEPH, OR 4.30 (1.29–14.35), as did COPD OR 3.17 (2.13–4.73), HF OR 2.52 (1.76–3.63) and AF OR 2.42 (1.71-3.42). Hypothyroidism was not associated with CTEPH. Although increased, the risk of systemic lupus erythematosus was not statistically significant, OR 2.79 (0.81–9.56) (Table 5). The sensitivity analysis using patients matched on year of birth and gender showed consistent findings but a significantly increased risk of OR 3.66 (1.06–12.65) for systemic lupus erythematosus and a decreased risk of OR 0.60 (0.41–0.87) for asthma (Supplementary Table 1). The sensitivity analyses changing the index date to 90 days before the original index day and stratifying the main results by VTE type, that is, DVT and pulmonary embolism, showed consistent results (Supplementary Tables 2 and 3).
Discussion
This study investigated risk factors for CTEPH in patients with VTE. The novel feature of our study is that we included DVT as opposed to just pulmonary embolism as in other studies. In this population-based longitudinal study with more than 10 years of observation of patients with first VTE and thorough search for CTEPH, we estimated an overall incidence rate of CTEPH of 3.5 (3.1–3.9) per 1000 person-years, with a peak in the 91 to 182 days following the first VTE. The overall cumulative incidence of CTEPH in the 10 years after the first pulmonary embolism was 3.3%, and following a first DVT was 1.3%. The probability of CTEPH is higher in those with a first or subsequent pulmonary embolism, but the risk of CTEPH is also present in those with a first DVT only, since 21.2% of patients with CTEPH were not found to have any history of pulmonary embolism.
Mortality rates for those with CTEPH at all time points were significantly greater compared with patients without CTEPH and peaked in the 90 days following the CTEPH diagnosis. This peak may indicate a higher prevalence of CTEPH in older ages and/or in patients with a high prevalence of associated medical conditions, and that those patients are less likely to be referred to one of the specialised centres for diagnostic workup and treatment.8,21
We found an association between the development of CTEPH and a first pulmonary embolism as the clinical manifestation of the first VTE and also with recurrent DVT and pulmonary embolism. We also found an association between splenectomy and CTEPH, as documented in previous studies.13,15 Our data indicate that COPD, AF and HF predict the risk of developing CTEPH.
It is known from post-mortem studies that pulmonary thrombosis is a common feature of COPD, particularly in the most damaged sections of the lung. This may not be diagnosed in life, where the diagnosis is more likely to be an acute exacerbation of COPD.
AF is associated with atrial clot which can lead to pulmonary embolism, particularly in the absence of therapeutic anticoagulation. Adherence in AF patients is low after the first 2 years of therapy. In addition there is a common association between ischaemic heart disease (IHD) and CTEPH. The IHD may lead to AF. Finally, there is a higher incidence of AF in PH, which is probably an association rather than a direct pathophysiological consequence and may physiologically be related to increased right atrial pressure. HF is associated with AF and stasis in the heart and circulation, both of which can predispose to the development of VTE.
Comparison with other studies
The overall probability of CTEPH following a pulmonary embolism in our study is within the range of the risk of CTEPH in other studies among patients with an acute pulmonary embolism episode of 0.4% to 8.8% after 2 to 10 years of observation.5,6,8,11,22–26
The overall 2-year mortality from the date of the first CTEPH diagnosis between 2001 and 2012 was 26.8% in our study, compared with approximately 23% in non-operated CTEPH in the UK National Audit of Pulmonary Hypertension (NAPH) for patients diagnosed after 2009 with CTEPH.27 The discrepancy between our data and the NAPH may in part be due to an older age of our CTEPH patients (median age 72 years) compared with a mean age of 59 to 60 years in the NAPH.27 It appears that younger and healthier patients with a potential diagnosis of CTEPH are preferentially sent to specialised centres for diagnostic workup and the availability of disease targeted drug therapy in recent years. This may indicate that some patients with advanced and severe CTEPH are not referred to specialist PH secondary care centres, thus being denied early initiation of CTEPH-specific treatment. Unlike previous studies, we did not find an association with hypothyroidism.13
Strengths and limitations
In those cases where information from right heart catheterisation was not available, a robust case-ascertainment algorithm was developed to identify CTEPH in patients with a first VTE using hospital discharge diagnoses, in-hospital procedures, non-invasive pulmonary artery pressures and specific medication use. The algorithm for ascertainment of CTEPH was initially based on published guidelines and expert clinical input. The validity of the algorithm was determined by a manual review of clinical notes available for a subset of potential CTEPH cases and revealed a sensitivity of 85.3%, and a specificity of 100% when the manual review process was integrated in the algorithm. However, we cannot rule out that the cohort might contain some patients with forms of PH other than CTEPH (e.g. pulmonary arterial hypertension, PH associated with heart or lung disease).
The risk factors have also been found in a sensitivity analysis using age and gender-matched controls in contrast to GP practice-matched controls in the main analysis and in a second sensitivity analysis using a different index day (i.e. moving the day of CTEPH diagnosis 90 days forward).
As in all observational studies, unmeasured confounding or hidden bias might exist. Because of the retrospective nature of this study, there were no uniform criteria for the diagnosis of CTEPH. Instead, an algorithm was developed to ascertain CTEPH from different data sources including in-hospital procedures such as pulmonary endarterectomies, hospital discharge diagnoses obtained from the HES, diagnoses and pulmonary artery pressure derived from clinical notes, and use of PH-specific medications.
PH-specific medications other than oral anticoagulants must only be prescribed by designated PH services in the UK. Furthermore, secondary care information is not systematically recorded and/or provided in a standardised form to GPs. Often, specialist letters are sent as PDF-images to GPs which are not accessible for electronic free text search in CPRD. In such cases, PH-specific medications may not be found, and so cases of CTEPH may be missed, leading to an underestimation of CTEPH and of CTEPH treatment.
We have estimated the cumulative incidence of CTEPH in a cohort with first VTE and without active cancer. Therefore, our incidence estimate is not generalisable to the incidence of CTEPH in the population, as CTEPH resulting from clinically undetected VTE was not included in our study population. Approximately 30% of patients with CTEPH have no history of VTE.13
In-hospital procedures, for example pulmonary endarterectomy or lung transplant, are likely to be recorded with respective procedure codes. The degree of under-recording of such procedures has not been estimated.
CTEPH may have an insidious onset and therefore it may take several months to develop and for the diagnosis to be established. During the workup to establish the diagnosis of CTEPH, patients often undergo a number of investigations including echocardiogram, ventilation-perfusion lung scanning and right heart catheterisation. The day of the established diagnosis will therefore not reflect the onset of CTEPH. However, to determine when the CTEPH became clinically apparent (index day), all cases of CTEPH were manually reviewed for symptoms which may have triggered the diagnostic workup. In addition, a sensitivity analysis using a 90-day shifted index day showed consistent results.
The clinical picture of CTEPH is not different from PH. To increase the likelihood of us detecting CTEPH and not PH from other causes we restricted the study cohort to patients with a first VTE. The fact that AF, left heart disease and COPD were found to be associated with CTEPH could indicate CTEPH cases in our study included PH from other causes (PH group 2 and 3), that VTE is an intermediate factor between the risk factors and CTEPH, or that patients with AF, HF and COPD could have PH due to left heart disease or lung disease with coincidental finding of perfusion defects and misdiagnosis of pulmonary embolism. The latter would have resulted in the misclassification of pulmonary embolism. However, the association between AF, HF and COPD and CTEPH was consistent in patients with a first DVT and patients with a history of pulmonary embolism.
Clinical implications and conclusions
This study is unique in that it uses a clinical guidelines-based algorithm to identify CTEPH patients from a primary care database linked with hospital and mortality data. CTEPH develops after pulmonary embolism but also, less frequently, after a diagnosis of DVT. COPD, AF and HF are associated with an increased risk of CTEPH after VTE. Awareness of frequent risk factors for CTEPH may increase the rate of diagnosis in primary and secondary care. Subsequent referrals to specialised centres for confirmation of CTEPH and initiation of CTEPH-specific treatment may improve prognosis.
Supplementary Material
Conflict of interest
C.M. and C.W. are employees of the Institute for Epidemiology, Statistics and Informatics GmbH. The Institute for Epidemiology, Statistics and Informatics GmbH has received grants from Bayer, Bristol-Myers Squibb, CSL Behring, and Merz Pharma outside the submitted work. C.M. has received personal fees from Boehringer Ingelheim outside the submitted work. A.C. reports grants and personal fees from Bayer AG, Bristol-Myers Squibb, Daiichi-Sankyo and Pfizer, and personal fees from Boehringer Ingelheim, Jannsen, Johnson and Johnson, Ono Pharmaceuticals, Portola, Sanofi, and X01 outside the submitted work; S.T. is an employee of Bayer Pharma AG, the sponsor of the study, and owns stock in Bayer AG; A.P. reports personal fees from Bayer during the conduct of the study; there are no other competing interests.
Funding
This study was initially funded by Bayer AG.
References
- 1.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37(1): 67–119. [DOI] [PubMed] [Google Scholar]
- 2.Kim NH, Delcroix M, Jenkins DP, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2013; 62(25 Suppl): D92–D99. [DOI] [PubMed] [Google Scholar]
- 3.Lang IM, Simonneau G, Pepke-Zaba JW, et al. Factors associated with diagnosis and operability of chronic thromboembolic pulmonary hypertension. A case-control study. Thromb Haemost 2013; 110(1): 83–91. [DOI] [PubMed] [Google Scholar]
- 4.Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): Results from an international prospective registry. Circulation 2011; 124(18): 1973–1981. [DOI] [PubMed] [Google Scholar]
- 5.Becattini C, Agnelli G, Pesavento R, et al. Incidence of chronic thromboembolic pulmonary hypertension after a first episode of pulmonary embolism. Chest 2006; 130(1): 172–175. [DOI] [PubMed] [Google Scholar]
- 6.Klok FA, van Kralingen KW, van Dijk AP, et al. Prospective cardiopulmonary screening program to detect chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Haematologica 2010; 95(6): 970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marti D, Gomez V, Escobar C, et al. [Incidence of symptomatic and asymptomatic chronic thromboembolic pulmonary hypertension]. Arch Bronconeumolog 2010; 46(12): 628–633. [DOI] [PubMed] [Google Scholar]
- 8.Miniati M, Monti S, Bottai M, et al. Survival and restoration of pulmonary perfusion in a long-term follow-up of patients after acute pulmonary embolism. Medicine (Baltimore) 2006; 85(5): 253–262. [DOI] [PubMed] [Google Scholar]
- 9.Ribeiro A, Lindmarker P, Johnsson H, et al. Pulmonary embolism: One-year follow-up with echocardiography doppler and five-year survival analysis. Circulation 1999; 99(10): 1325–1330. [DOI] [PubMed] [Google Scholar]
- 10.Yang S, Yang Y, Zhai Z, et al. Incidence and risk factors of chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. J Thorac Dis 2015; 7(11): 1927–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004; 350(22): 2257–2264. [DOI] [PubMed] [Google Scholar]
- 12.Ende-Verhaar YM, Cannegieter SC, Vonk Noordegraaf A, et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: A contemporary view of the published literature. Eur Resp J 2017; 49(2): pii: 1601792. [DOI] [PubMed] [Google Scholar]
- 13.Bonderman D, Wilkens H, Wakounig S, et al. Risk factors for chronic thromboembolic pulmonary hypertension. Eur Resp J 2009; 33(2): 325–331. [DOI] [PubMed] [Google Scholar]
- 14.Lang IM. Chronic thromboembolic pulmonary hypertension – not so rare after all. N Engl J Med 2004; 350(22): 2236–2238. [DOI] [PubMed] [Google Scholar]
- 15.Jais X, Ioos V, Jardim C, et al. Splenectomy and chronic thromboembolic pulmonary hypertension. Thorax 2005; 60(12): 1031–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonderman D, Jakowitsch J, Adlbrecht C, et al. Medical conditions increasing the risk of chronic thromboembolic pulmonary hypertension. Thromb Haemost 2005; 93(3): 512–516. [DOI] [PubMed] [Google Scholar]
- 17.Martinez C, Cohen AT, Bamber L, et al. Epidemiology of first and recurrent venous thromboembolism: A population-based cohort study in patients without active cancer. Thromb Haemost 2014; 112(2): 255–263. [DOI] [PubMed] [Google Scholar]
- 18.Montani D, Gunther S, Dorfmuller P, et al. Pulmonary arterial hypertension. Orphanet J Rare Dis 2013; 8: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(25 Suppl): D34–D41. [DOI] [PubMed] [Google Scholar]
- 20.Task Force for D, Treatment of Pulmonary Hypertension of European Society of C, European Respiratory S, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Resp J 2009; 34(6): 1219–1263. [DOI] [PubMed]
- 21.Condliffe R, Kiely DG, Gibbs JS, et al. Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Resp Crit Care Med 2008; 177(10): 1122–1217. [DOI] [PubMed] [Google Scholar]
- 22.Dentali F, Donadini M, Gianni M, et al. Incidence of chronic pulmonary hypertension in patients with previous pulmonary embolism. Thromb Res 2009; 124(3): 256–258. [DOI] [PubMed] [Google Scholar]
- 23.Guerin L, Couturaud F, Parent F, et al. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Prevalence of CTEPH after pulmonary embolism. Thromb Haemost 2014; 112(3): 598–605. [DOI] [PubMed] [Google Scholar]
- 24.Korkmaz A, Ozlu T, Ozsu S, et al. Long-term outcomes in acute pulmonary thromboembolism: The incidence of chronic thromboembolic pulmonary hypertension and associated risk factors. Clin Appl Thromb Hemost 2012; 18(3): 281–288. [DOI] [PubMed] [Google Scholar]
- 25.Poli D, Grifoni E, Antonucci E, et al. Incidence of recurrent venous thromboembolism and of chronic thromboembolic pulmonary hypertension in patients after a first episode of pulmonary embolism. J Thromb Thrombolysis 2010; 30(3): 294–299. [DOI] [PubMed] [Google Scholar]
- 26.Surie S, Gibson NS, Gerdes VE, et al. Active search for chronic thromboembolic pulmonary hypertension does not appear indicated after acute pulmonary embolism. Thromb Res 2010; 125(5): e202–e205. [DOI] [PubMed] [Google Scholar]
- 27.Health and Social Care Information Centre. National Audit of Pulmonary Hypertension 2014. Fifth Annual Report: Key findings from the National Audit of Pulmonary Hypertension for the United Kingdom, Channel Islands, Gibraltar and Isle of Man. Report for the audit period April 2013 to March 2014. 2014. Available from: https://digital.nhs.uk/catalogue/PUB17264 (accessed 15 September 2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.