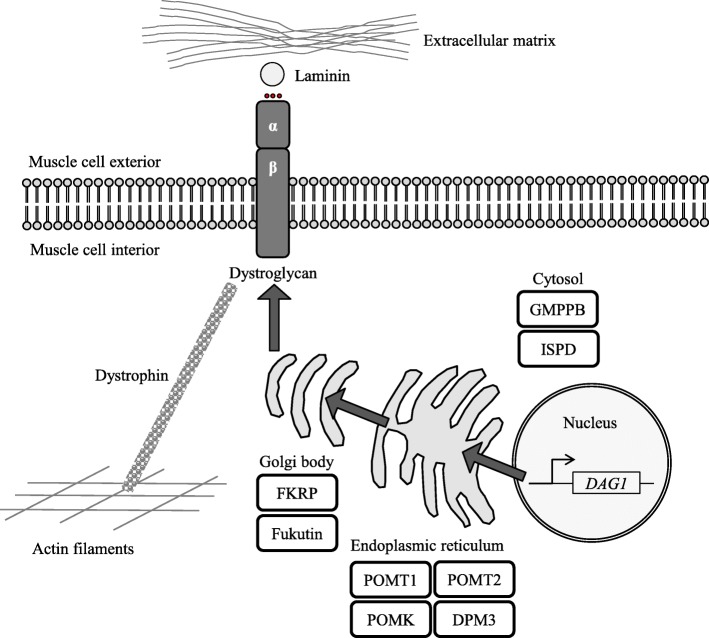
Fig. 1.
Localisation of the proteins involved in the glycosylation of α-dystroglycan. Only the encoded proteins of the genes identified as harbouring suspected pathogenic variants in the MYO-SEQ project are shown. DAG1 is transcribed and translated into α-dystroglycan and β-dystroglycan subunits. As the proteins are processed through the endoplasmic reticulum and Golgi body to the muscle cell membrane (pathway indicated by grey arrows), GMPPB, POMT1, POMT2, POMK, ISPD, DPM3, FKRP and fukutin all contribute to the correct glycosylation of the α-subunit. The glycosylation of α-dystroglycan is required for interactions with extracellular matrix components; the dystroglycan complex as a whole thus acts as an anchor between the extracellular matrix and the intercellular actin cytoskeleton