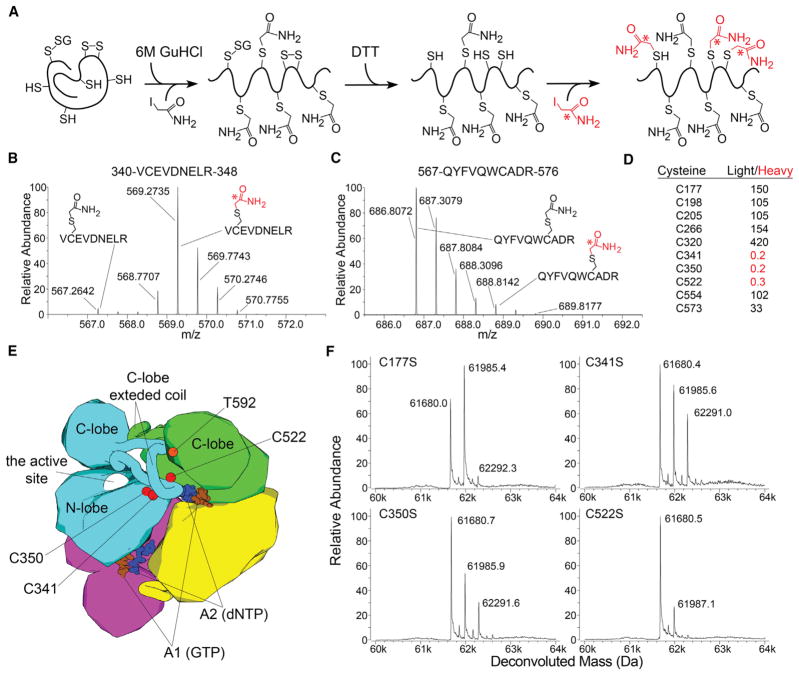
Figure 2. Identification of the Redox-Active Cysteines by Mass Spectrometry.
(A) Experimental procedure for quantifying cysteine oxidation in the protein treated with oxidized glutathione. The quantitative proteomics analysis relies on the differential labeling of the protein with the light and heavy forms of iodoacetamide.
(B–D) All cysteine-containing peptides were observed to fall into two distinct classes: peptides predominantly labeled with the heavy iodoacetamide (B) and peptides predominantly labeled with the light form (C). (D) Typical light-to-heavy abundance ratios measured for peptides containing each of the 10 cysteines in SAMHD1.
(E) Cartoon depiction of the SAMHD1 tetramer fully loaded with allosteric ligands as observed by X-ray crystallography (PDB: 4BZC). The three redox-active cysteines reside near the tetramerization interface, the allosteric binding sites, and the T592 residue. The proximity of C341 and C350 to each other favors formation of the intramolecular disulfide bond.
(F) Effect of cysteine mutations on SAMHD1 glutathionylation as evaluated by intact-mass analysis (ESI-TOF).
See also Figures S2 and S3.