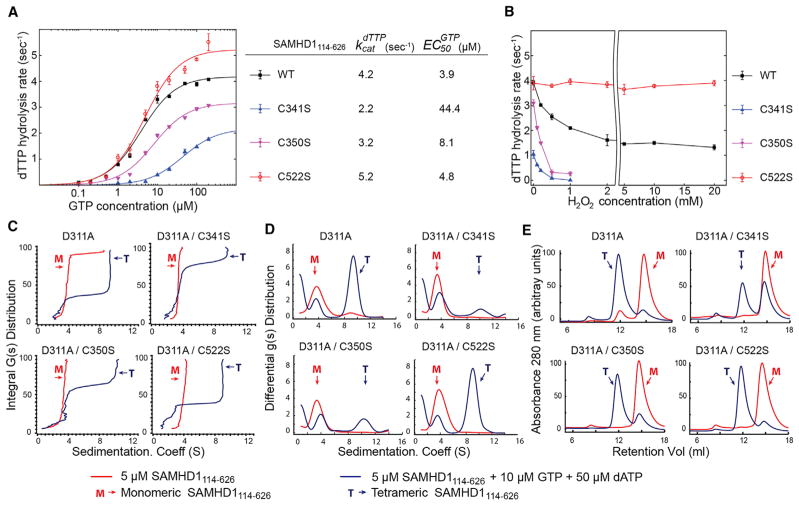
Figure 3. Effects of Cysteine Mutations on the dNTPase Activity and Tetramerization of SAMHD1114–626.
(A) dNTPase activity of SAMHD1 mutants evaluated as a function of GTP concentration using an NMR-based dNTPase assay. kcat and EC50GTP parameters were determined by non-linear fitting of the data to the simple EC50 equation.
(B) H2O2 sensitivity of SAMHD1 cysteine mutants. After 3 min of incubation with increasing concentrations of H2O2, the dTTP hydrolysis rate was measured in the presence of 50 μM GTP.
(C and D) Analytical ultracentrifugation studies of the catalytically inactive SAMHD1 variants. Sedimentation velocity runs were performed with protein alone (red traces) and in the presence of nucleotides (blue traces). Sedimentation velocity analysis showing diffusion-corrected van Holde-Weischet integral G(s) distributions (C) and differential g(s) distributions (D) obtained from the parametrically constrained spectrum analysis.
(E) Size-exclusion chromatography analysis of SAMHD1 tetramerization is in agreement with the AUC results. n = 2, where n represents the number of independent measurements of the rate. Error bars denote SD.