Fig. 2.
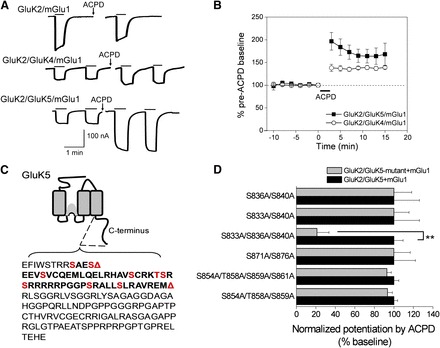
Potentiation of heteromeric KARs by group I mGlu activation. (A) Whole-cell currents were recorded from oocytes expressing GluK2/mGlu1, GluK2/GluK4/mGlu1, or GluK2/GluK5/mGlu1. Following application of 100 μM ACPD (a nonspecific mGlu activator), steady-state activation of the heteromeric receptors by AMPA (30 μM) was potentiated, but not steady-state activation of the homomeric KAR by domoic acid (10 μM). (B) Time-dependent potentiation of GluK2/GluK4/mGlu1 and GluK2/GluK5/mGlu1 AMPA currents by mGlu1 activation with ACPD (n = 5 and n = 16, respectively). (C) Schematic showing the GluK5 C-terminal domain. The first red triangle indicates the location of an inserted stop codon (Δ837) and the second red triangle indicates the position of a more distal stop codon (Δ884). The red residues indicate consensus PKC phosphorylation sites. (D) Alanine mutagenesis of the C-terminal serines and threonines between 833 and 883 revealed that only the triple GluK5 mutant (GluK5-S833A/S836A/S840A) resulted in a significantly reduced mGlu1-mediated potentiation (n ≥ 4; each mutant combination was evaluated against a separate set of oocytes expressing wild-type receptors; **P < 0.001; t test). From Rojas et al. (2012).
