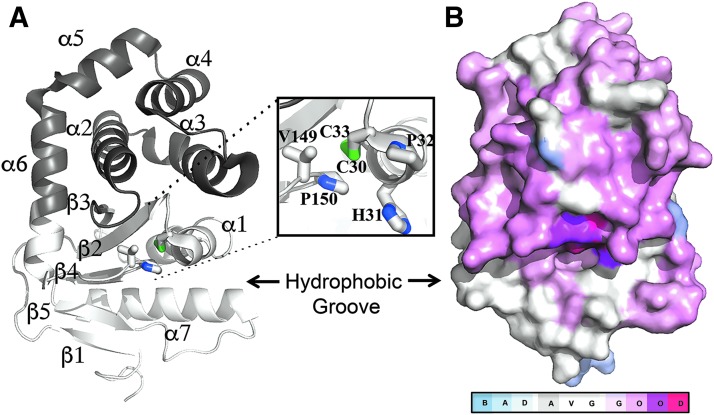
FIG. 1.
Escherichia coli DsbA structure and sequence conservation. (A) Ribbon diagram of EcDsbA [PDB 1FVK (24)]. The TRX-fold and inserted helical domain are shown in light and dark gray, respectively. Secondary structural features are labeled, and the catalytic disulfide bond is shown in green. Inlet shows a close-up view of the active site of EcDsbA encompassing a Cys30-Pro31-His32-Cys33 redox-active motif and the adjacent cis-proline loop (V149-cisPro150). (B) Amino acid sequence conservation in diverse bacterial DsbA prototypes. The conservation scores obtained from an Expresso T-Coffee multiple sequence alignment of 20 DsbA protein sequences are depicted on the surface of EcDsbA by using a color gradient, from blue (poorly conserved), through to white (reliable conservation) and red (well conserved). EcDsbA, Escherichia coli disulfide bond; TRX, thioredoxin.