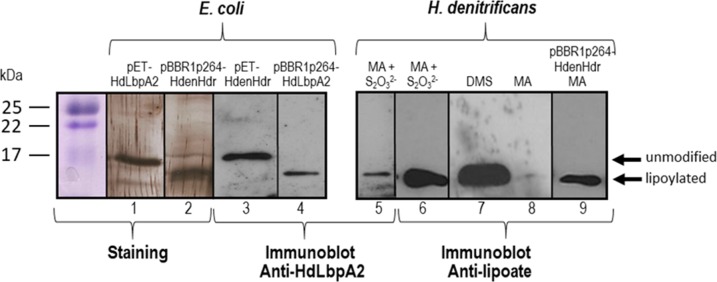
Figure 4. SDS-PAGE and Western blot of HdLbpA2.
Lanes 1, 2, 3 and 4 were loaded with 20 ng of pure recombinant HdLbpA2 stemming from pET- or pBBR1p264-based expression as indicated. Lanes 5 to 8 were loaded with extracts (15–20 μg protein) H. denitrificans wildtype grown on methylamine plus thiosulfate (MA + S2O32-), dimethylsulfide (DMS) and methylamine (MA). Lane 9 was loaded with extract (17.5 μg protein) of H. denitrificans expressing plasmid-encoded genes dsrEhdrC1B1AhyphdrC2B2hyplbpA from a constitutive promoter. Lanes 1 and 2 show silver stained gels. Western blots were developed with antiserum against HdLbpA2 or with anti-lipoic acid antibody as indicated.