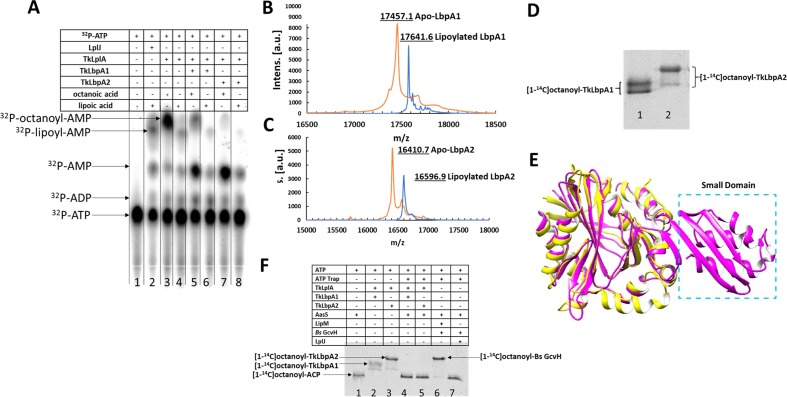
Figure 7. Enzymatic role of LplA from sulfur oxidizing bacteria in lipoylation/octanoylation of Lbp proteins.
(A) TLC analysis of products formed from [α-32P]ATP. Both synthesis of lipoyl/octanoyl-5’-AMP and transfer of the lipoyl/octanoyl moiety to the acceptor proteins LbpA1 and LbpA2 are shown respectively. Addition of an acceptor protein results in consumption of the acyl adenylate intermediates and production of AMP. TkLplA, TkLbpA1 and TkLbpA2 denote LplA, LbpA1 and LbpA2 proteins from Thioalkalivibrio sp. K90mix, respectively. (B and C) MALDI mass spectrometric analysis of lipoylation of TkLbpA1 and TkLbpA2 respectively catalyzed by LplA from Thioalkalivibrio sp. K90mix. The mass of the lipoylated LbpA1 (17641.6 Da) and lipoylated LbpA2 form (16596.9 Da) agree well the calculated values. The change in mass upon modification (calculated for lipoyl modification, 188 Da; observed, 184.5 Da for LbpA1 and 186.2 Da for LbpA2 respectively) is within the accuracy of the instrument utilized. Intens., intensity; a.u., arbitrary units. (D) Activity of TkLplA (KEGG: TK90_0642) on the two lipoate-binding proteins. [1-14C]octanoic acid was used as the substrate. Reactions were incubated at 37°C for 1 hr and loaded on a 15% SDS-PAGE gel. The [1-14C]octanoyl-Lbp proteins were detected by autoradiography. Lane 1, LbpA1 from Thioalkalivibrio sp. K90mix (TK90_0638); lane 2, LbpA2 from Thioalkalivibrio sp. K90mix (TK90_0640). (E) Superimposition model of from Thioalkalivibrio sp. K90mix LplA (yellow) obtained by threading on the structure of E. coli LplA (magenta, PDB 3A7R). The small domain of E. coli LplA was denoted by blue dotted box. (F) Detection of [1-14C]octanoyl transfer from [1-14C]octanoyl-ACP to lipoate-binding proteins (Lbps). The [1-14C]octanoyl-ACP was synthesized in situ from [1-14C]octanoate, V. harveyi AasS acyl ACP synthetase, ATP and E. coli holo-ACP, whereas formation, if any, of [1-14C]octanoyl-Lbp should require apo-Lbp, octanoyl transferase and [1-14C]octanoyl-ACP. Note that the residual ATP remaining from the AasS reaction was depleted by use of an ATP trap (2 units of hexokinase plus 10 mM D-glucose to convert ATP to glucose-6-phosphate and ADP). Each reaction contained ATP, [1-14C]octanoate, and E. coli holo-ACP. Lane 1, [1-14C]octanoyl-ACP; Lane 2, positive control LbpA1 (TK90_0638) in the presence of ATP and LplA (TK90_0642); Lane 3, positive control LbpA2 (TK90_0640) in the presence of ATP and LplA (TK90_0642); Lane 4, LbpA1with AasS coupled reaction in the presence of LplA and ATP trap; Lane 5, LbpA2 with AasS coupled reaction in the presence of LplA and ATP trap; Lane 6, B. subtilis GcvH protein with AasS coupled reaction in the presence of a real octanoyltransferase LipM from B. subtilis and ATP trap.
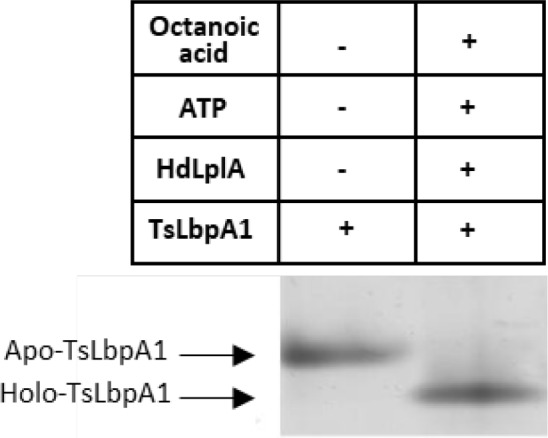
Figure 7—figure supplement 1. Mobility shift assay for analysis of in vitro octanoylation catalyzed by HdLplA from Hyphomicrobium denitrificans (Hden_0686) using LbpA1 from Thiorhodospira sibirica (TsLbpA1, ThisiDRAFT_1533) as acceptor.