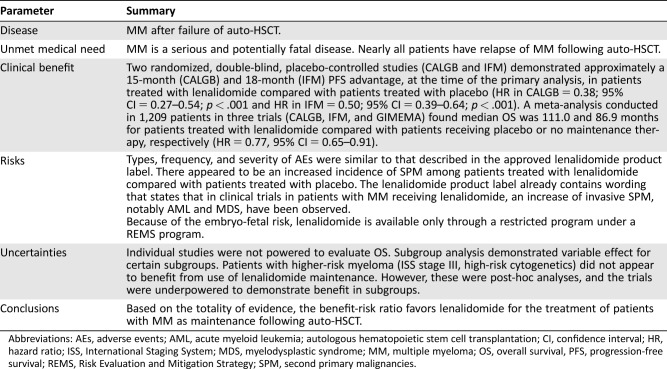
Table 4. U.S. Food and Drug Administration benefit‐risk assessment.
Abbreviations: AEs, adverse events; AML, acute myeloid leukemia; autologous hematopoietic stem cell transplantation; CI, confidence interval; HR, hazard ratio; ISS, International Staging System; MDS, myelodysplastic syndrome; MM, multiple myeloma; OS, overall survival, PFS, progression‐free survival; REMS, Risk Evaluation and Mitigation Strategy; SPM, second primary malignancies.