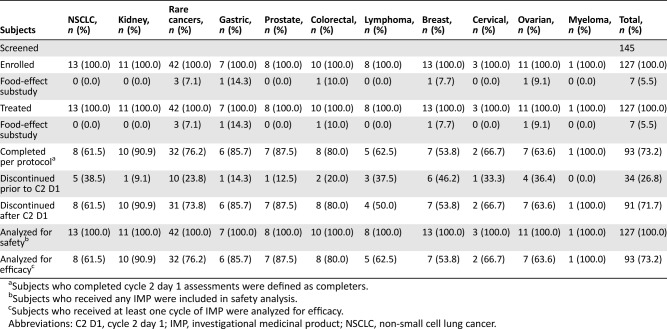
Table 3. Subject disposition during dose expansion (stage 2).
Subjects who completed cycle 2 day 1 assessments were defined as completers.
Subjects who received any IMP were included in safety analysis.
Subjects who received at least one cycle of IMP were analyzed for efficacy.
Abbreviations: C2 D1, cycle 2 day 1; IMP, investigational medicinal product; NSCLC, non‐small cell lung cancer.