Abstract
Background
Both heart failure (HF) symptoms and self-care are associated with patient outcomes. Although it is thought that symptoms drive self-care, there is limited evidence to support this assumption over time.
Aims
Determine whether patterns of physical symptoms are significantly associated with HF self-care over time.
Method
Latent mixture analysis was used to identify sub-groups based on physical symptoms of dyspnea, sleepiness and edema (using the HF Somatic Perception and Epworth Sleepiness Scales). Growth modeling was used to determine if symptom sub-groups were associated with self-care behaviors (using the Self-care in HF Index) over 6 months. Socio-demographic and clinical variables predicting likelihood of sub-group membership were identified using logistic regression.
Results
The sample (n=146) was on average 57 years old, 70% were men and 59% had class III/IV HF. Two symptom sub-groups were identified (entropy=0.91); a high symptom group (n=24(16%)) with no significant change in symptoms over time (high sustained), and low symptom group (n=122(84%)) with no significant change in symptoms over time (low sustained). The high sustained group was associated significantly with better self-care behaviors at baseline and over 6 months. Women (OR=3.67, p=0.023) and patients with more depressive symptoms (OR=1.16, p=0.015) were more likely to be in the high sustained symptom group. Those treated with a renin-angiotensin-aldosterone system agent were less likely to be in the high symptom group (OR=0.17, p=0.015).
Conclusion
Patients bothered more by symptoms are consistently more engaged in self-care behaviors over time. Results of this study support symptoms as an important driver of self-care behaviors.
Keywords: heart failure, symptoms, self-care, symptom management, depression
Introduction
Heart failure (HF) is a growing global problem, affecting an estimated 26 million people worldwide and is the leading cause of hospital admission for older adults in the US.1,2 Exacerbation of physical symptoms such as dyspnea is the primary reason patients with HF seek care often leading to burdensome and costly hospitalizations.3 Symptoms are of primary concern for clinicians and patients as symptoms are associated with mortality risk, strongly affect quality of life and are used to evaluate progression of HF and guide HF therapies.4,5 Due to the importance of physical symptoms in HF, self-care behaviors to prevent and monitor symptoms (maintenance) and decision-making behaviors addressing symptoms once detected (management) are cornerstones of HF management.6 More engagement in self-care behaviors is associated with fewer hospitalizations, better quality of life and lower mortality.7 Although assessment of physical symptoms and self-care are essential aspects of HF management, little is known about how symptoms and self-care are related to one another particularly over time.
Studies that have examined the relationship between physical symptoms and HF self-care have yielded inconsistent results. For example, HF symptoms have been shown to be both positively and negatively associated to self-care behaviors. Lee et al.8 and Rockwell & Riegel 9 demonstrated higher levels of HF symptoms associated with increased HF self-care behaviors while Graven and colleagues10 describe less engagement in HF self-care associated with worse HF symptoms. Similarly, patients less engaged with adherence behaviors, such as eating a low salt diet,11 have been shown to have more severe symptoms compared to those with higher levels of such behaviors. Furthermore, patients often experience physical symptoms and practice self-care behaviors in distinct patterns which have been shown to provide important information regarding mortality and clinical event risk.7,12 For example, Lee et al.12 describe three distinct physical and psychological symptom patterns (mild, moderate, and severe) that were associated with 1 year event free survival. The relationship between patterns of physical symptoms and self-care, however, has yet to be explored. Thus, how HF symptoms and HF self-care behaviors influence one another remains unclear.
Due to the importance of both symptoms and self-care in affecting patient outcomes, understanding the relationship between HF symptoms and self-care behaviors is critical in advancing the care of patients with HF and the understanding of how and why patients engage in self-care behaviors. One limiting factor in elucidating the important relationship between symptoms and self-care is the lack of longitudinal studies in this area. The vast majority of studies are cross-sectional, hindering the ability to examine how symptom are related to self-care over time. Without longitudinal studies we cannot begin to untangle the temporal or directional association of symptoms and self-care in HF. The purpose of this longitudinal study is to determine 1) whether patterns of HF symptoms are significantly associated with corresponding patterns in self-care behaviors over 6 months and 2) determine socio-demographic and clinical predictors of symptom patterns. The results of this study will provide essential information that may enhance our understanding of HF self-care and physical symptoms to guide the development of interventions focused on improving outcomes for patient with HF.
Methods
Design, Setting, Sample
In this analysis, a sample of 146 participants with symptomatic HF (NYHA Class II-IV) were recruited for a longitudinal study from a community-based HF clinic associated with an academic medical center. Details of the original study have been previously described.8 All participants were being optimally managed by a cardiologist specializing in advanced HF. At a clinic appointment, all participants were provided written informed consent presented by a member of the study team not associated with their care. The study was approved by the Institutional Review Board associated with the academic medical center. The investigation conforms to the principles outlined in the Declaration of Helsinki.13 Socio-demographic information was collected at enrollment. Additionally, at enrollment, three and six months, participants completed a survey of symptoms (physical and emotional) and engagement of self-care behaviors. Surveys were completed in person during their clinic visit, by mail, or by telephone per the participants’ preference. Additional clinical data associated with HF was abstracted at each time point (enrollment, 3 and 6 months). Attrition at 6 months was 9.3%.
Measures
HF symptoms
Dyspnea was measured with the 6-item dyspnea subscale of the Heart Failure Somatic Perception Scale (HFSPS dyspnea).14 The HFSPS asks about how much the participant was bothered by HF symptoms related to dyspnea during the last week and provides six response options ranging from 0 (not at all) to 5 (extremely bothersome). The HFSPS dyspnea has excellent internal consistency (alpha = 0.89) concordant validity with the KCCQ functional limitations scale (r = 0.53) and independently predicts HF-related clinical events (per point HR = 1.031, p=0.031).14
Patient-perceived edema was measured with the 3-item edema subscale of the HFSPS (HFSPS edema).14 The HFSPS edema asks about how much the participant was bothered by feet swelling, tightness of shoes and weight gain. The HFSPS edema provides six response options ranging from 0 (not at all) to 5 (extremely bothersome) and has good internal consistency (alpha = 0.75).14
Wake disturbance was measured with the Epworth Sleepiness Scale (ESS). The ESS asks participants to rate how likely they are to fall asleep in 8 different situations. The rating range from 0 (never) to 3 (high chance).15 Scores range from 0–24 with higher values indicating more daytime wake disturbance. The ESS correlates with the Multiple Sleep Latency Test and has been shown to be responsive to treatment effects of continuous positive airway pressure on sleep propensity.15,16
HF self-care behaviors
Heart failure self-care was measured using the Self-care of HF Index (SCHFI v.6) self-care maintenance and self-care management scales with higher values indicating better self-care (standardized scores of 0–100).17 The 10 item self-care maintenance scale uses 4 response options (never, sometime, frequently, always) and has adequate internal consistency.18 The SCHFI self-care management scale has 6 items using a 4 or 5 point scale with adequate internal consistency.18 Self-care of HF Index scores less than 70 are considered inadequate self-care.19
Predictors of symptom patterns
Symptoms of depression were measured using the 9-item Patient Health Questionnaire (PHQ9). The PHQ9 asks patients to assess nine depressive symptom with responses ranging from 0 (not at all) to 3 (nearly every day). A higher rating indicates more severe depressive symptoms with scores of 5, 10, 15 and 20 indicative of mild, moderate, moderately severe and severe depression respectively.20 The PHQ9 has been previously shown to be sensitive and specific for depression in HF with good reliability.21
Anxiety was measured using the 6-item anxiety sub-scale of the brief symptom inventory (BSI-anxiety). The BSI asks patients to assess their feelings over the previous 7 days and offers 5 response options ranging from 0 (not at all) to 4 (extremely). The sub-scale score is determined by adding the responses and dividing by the total number of items answered. Higher scores on the BSI-anxiety indicate more anxiety.22 The BSI-anxiety has been shown to be valid and reliable in patients with HF.23
Data Analysis
Proportions, means and standard deviations were used to describe the sample as a whole. The Student’s t test with unequal variances, Mann-Whitney U test, Chi-square or Fisher’s exact test were used to describe differences between groups. Hedge’s g was also calculated as the effect size to quantify the magnitude of difference in symptoms. The overall approach to examine the association of physical symptom patterns with self-care over time was first to identify naturally-occurring sub-groups based on a cluster of physical symptoms (dyspnea, wake disturbance and edema). The physical symptoms were chosen because they approximate hallmark HF symptoms and represent both left- and right-sided HF. The next step was to associate the physical symptom sub-groups with baseline values (intercepts) and/or changes in self-care over time (slopes). The final step was to predict symptom group membership using sociodemographic and clinical variables.
Our original analytic strategy was to focus on change in symptoms over time. Since there was no significant change in symptoms over time, however, we reverted to latent class mixture analysis (LCMA) (Mplus v7.4, Los Angeles, CA) to identify naturally-occurring groups. The sub-groups were identified based on baseline symptom data because there was no significant variation in symptom slopes indicating the baseline symptoms were representative of the patient experience over 6 months. Alternative models (e.g. 2 classes vs 3 classes) were compared using posterior probabilities (>90%), model convergence (entropy near 1.0), the size of the observed classes (> 5%) and the Lo-Mendell-Rubin Likelihood Ratio Test (LMRT)(p<0.05).24 Latent growth modeling was then used to associate naturally-occurring symptom groups with intercepts (baseline values) and slopes (change over time) of self-care behaviors over time. Metrics of fit between estimated and observed data for the latent growth models were evaluated with the chi-square test of model fit (>0.05), comparative fit indices and Tucker-Lewis indices ≥ 0.95,25 root mean square errors of approximation <0.10,26 and standardized root mean square residuals <0.08.25 Results from LGM are provided in estimates similar to regression coefficients, standard error and p-values for the intercepts (i) and slopes (s).
Finally, clinical and socio-demographic variables predicting the likelihood of belonging to a physical symptom group were evaluated using multivariate backwards stepwise logistic regression with a p value of 0.2 for variable inclusion in the final model (Stata v14, College Station, TX). Logistic regression analyses are reported as odd ratios (OR) with p-values and 95% confidence intervals. P-values less than 0.05 are considered significant in this analysis. Socio-demographic (e.g. age, gender, race, marital status, education) and HF-related covariates (e.g. HF etiology, left ventricular diastolic diameter, ejection fraction, duration of HF, prescription of HF medications) as well as variables previously shown to influence symptoms and self-care (e.g. comorbidities, BMI, depression and anxiety) were included in the stepwise logistic regression analyses.
Results
The sample (n = 146) was 57 years old on average, 30% of participants were women and a majority self-identified Caucasian (86%) (Table 1). A majority of participants had non-ischemic cardiomyopathy (64%) and most experienced NYHA Class III/IV (59%) symptoms at enrollment. Dyspnea, wake disturbance, and patient-perceptions of edema did not change significantly over the course of 6 months as measures at three month intervals (Table 2). Overall, self-care maintenance behaviors were adequate (>70) at baseline and remained stable for the 6 month study period (i =70.3±1.4, p<0.001; s =0.9±0.6, p=0.112). Self-care management, on the other hand, was inadequate at baseline (<70) and did not improve significantly over the course of the study (i =61.2±1.9, p<0.001; s =0.02±0.9, p =0.987).
Table 1.
Characteristics of the Sample (n= 146)
| --------------Symptom Group-------------- | ||||
|---|---|---|---|---|
|
| ||||
| Sample (n = 146) | Sustained High (n = 24) | Sustained Low (n = 122) | p value | |
| Age (in years) | 57 ± 13.5 | 55.9 ± 3.3 | 57.7 ± 1.1 | 0.601 |
| Female | 44 (30.1) | 11 (45.8) | 33 (27.0) | 0.067 |
| Self-Identified Race | ||||
| Caucasian | 126(86.3) | 18 (75.0) | 108 (88.5) | 0.082 |
| Education | ||||
| High School or less | 45 (30.8) | 8 (33.3) | 37 (30.3) | 0.473 |
| Marital Status | ||||
| Married or living with partner | 94 (64.4) | 16 (66.7) | 78 (63.9) | 0.798 |
| Charlson Co-morbidity category | 0.378 | |||
| low (score of 1 or 2) | 93 (63.7) | 13 (54.2) | 80 (65.6) | |
| medium (score of 3 or 4) | 50 (34.2) | 11 (45.8) | 39 (32.0) | |
| high (score of 5 or more) | 3(2.1) | 0 | 3 (2.4) | |
| BMI | 32.0 ± 0.6 | 33.4 ± 1.9 | 31.7 ± 0.6 | 0.389 |
| Heart Failure Characteristics: | ||||
| NYHAIII/IV | 86 (58.9) | 18(75.0) | 68 (55.7) | 0.080 |
| EF% | 28.4 ± 11.6 | 31.0 ± 2.5 | 28.0 ± 1.1 | 0.350 |
| Primary Etiology | ||||
| Ischemic | 52 (35.6) | 5 (20.8) | 47 (38.5) | 0.109 |
| Systolic BP | 109.3 ± 13.9 | 112.1 ± 3.5 | 108.8 ± 1.2 | 0.384 |
| Aldosterone Antagonist | 66 (45.2) | 11 (45.8) | 55 (45.1) | 0.946 |
| ACE/ARB | 126 (86.3) | 16 (66.7) | 110 (90.2) | 0.002 |
| Beta Blocker | 134 (91.8) | 21 (87.5) | 113 (92.6) | 0.417 |
| Hemoglobin | 13.3 ± 2.0 | 12.8 ± 0.4 | 13.5 ± 0.2 | 0.134 |
| Serum Sodium | 138.6 ± 3.2 | 138.1 ± 0.7 | 138.6 ± 0.3 | 0.520 |
| Years with HF | 6.4 ± ±5.3 | 5.3 ± 1.0 | 6.6 ± 0.5 | 0.253 |
| Baseline HF Symptoms: | ||||
| Dyspnea | 7.3 ± 7.5 | 15.0 ± 1.5 | 5.8 ± 0.6 | <0.001 |
| Wake Disturbance | 7.9 ± 4.7 | 11.4 ± 1.2 | 7.2 ± 0.4 | <0.001 |
| Edema | 3.0 ± 3.8 | 10.1 ± 0.6 | 1.6 ± 0.2 | <0.001 |
| Baseline Self-care Behaviors: | ||||
| Self-care maintenance | 70.4 ± 16.1 | 75.8 ± 2.6 | 69.3 ± 1.5 | 0.036 |
| Self-care management | 63.9 ± 21.5 | 75 ± 3.8 | 61.7 ± 1.9 | 0.003 |
| Baseline Depression: | 7.0 ± 5.9 | 10.2 ± 1.3 | 6.4 ± 0.5 | 0.009 |
| Baseline Anxiety: | 0.5 ± 0.6 | 0.7 ± 0.1 | 0.5 ± 0.1 | 0.287 |
Abbreviations: BMI–body mass index; NYHA–New York heart association classification; EF–ejection fraction; BP–blood pressure; ACE–angiotensin converting enzyme inhibitor; ARB-angiotensin receptor blocker; HF–heart failure.
Table 2.
Physical Symptom Growth Model Parameters
| co-efficient (standard error) | t score | p value | |
|---|---|---|---|
| Dyspnea | |||
|
| |||
| intercept | 7.19 (0.62) | 11.55 | <0.001 |
| slope | −0.26 (0.27) | −0.97 | 0.332 |
|
| |||
| Wake Disturbance | |||
|
| |||
| intercept | 7.814(0.39) | 20.22 | <0.001 |
| slope | 0.24(0.15) | −1.62 | 0.106 |
|
| |||
| Edema | |||
|
| |||
| intercept | 3.02 (0.32) | 9.52 | <0.001 |
| slope | −0.07 (0.15) | −0.48 | 0.633 |
| For all models: Chi-squared test of model fit >0.05; RMSEA<0.08; CFI>0.95; TLI>0.95; SRMR<0.06 | |||
There was no significant change (slopes) in the physical symptoms over 6 months. Abbreviations: RMSEA – root mean square error of approximation; CFI/TLI-comparative fit indices/Tucker-Lewis indices, SRMR – standardized root mean-square residuals
Based on the LCMA, two distinct physical symptom groups were identified at baseline (LMRT: p = 0.008, posterior probabilities range: 0.92–0.99, entropy: 0.91). Considering the differentiating characteristics (Table 1) and no significant rate of change in the symptoms over time, the two groups were labeled high sustained symptoms (HSS) and low sustained symptoms (LSS). The HSS group comprised 16% of the sample (n=24) and had markedly worse dyspnea (hedges g=−1.36), wake disturbance (hedges g=−0.95) and edema (hedges g=−3.95) compared with the LSS group.
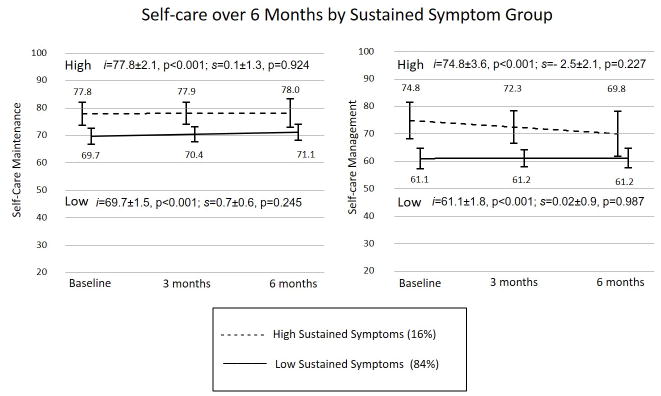
Differing patterns of self-care were identified as a function of the physical symptom group membership (HSS vs LSS). For patients belonging to the HSS group, self-care maintenance and management were significantly higher at baseline with sustained high levels of self-care over the 6 months (Figure 1). In contrast, patients belonging to the LSS group showed persistent lower levels of self-care over the 6 months.
Figure 1.
Compared by sustained symptom group (High vs Low), self-care maintenance and management differed significantly at baseline (intercept) with no significant difference between groups in the rate of change (slope) over time (Self-care maintenance by symptom group: intercept t=2.1, p=0.035, slope t=−0.3, p=0.754; Self-care management by symptom group: intercept t=3.0, p=0.003, slope t=−0.9, p=0.376). The high sustained symptom group was associated with higher self-care (maintenance and management) at baseline with persistently higher levels of self-care over 6 months. The low sustained symptom group was associated with lower self-care at baseline and consistently lower self-care over 6 months. Metrics of fit for latent growth models: Self-care maintenance: χ2 test of model fit =0.107; RMSEA=0.092; CFI=0.989; TLI=0.967; SRMR<0.019; Self-care management: χ2 test of model fit=0.539; RMSEA<0.001; CFI=1.000; TLI=1.019; SRMR<0.016.
Abbreviations: HSS – high sustained symptoms; LSS – low sustained symptoms; RMSEA – root mean square error of approximation; CFI/TLI-comparative fit indices/Tucker-Lewis indices, SRMR – standardized root mean-square residuals
There were unadjusted differences in the symptom groups with baseline depression (t = −2.78, p=0.009) and ACE inhibitor/ARB treatment (χ2=9.37, p=0.002). In multivariate logistic regression, however, the likelihood of being in the HSS group included being female, higher levels of depression and having an ACE inhibitor or ARB prescribed (Table 3). There were no significant differences in depression between women and men within the LSS group (p=0.168) or the HSS group (p=0.648).
Table 3.
Predictors of High Sustained Symptom Group
| Odds Ratio (CI) | p value | |
|---|---|---|
| Race | 0.34(0.09–1.33) | 0.121 |
| Gender (Female) | 3.67(1.19–11.27) | 0.023 |
| Anxiety | 0.45(0.15–1.32) | 0.144 |
| BMI | 1.06(0.99–1.14) | 0.083 |
| HF Etiology | 2.43(0.63–9.43) | 0.198 |
| ACE/ARB | 0.17(0.04–0.71) | 0.015 |
| Depression | 1.16(1.03–1.31) | 0.015 |
Factors entered into stepwise model included age, gender, married or living with partner vs living alone, Caucasian vs non-Caucasian, high school education or less vs more than high school, Charlson Co-morbidity Index, BMI, HF etiology, months since HF diagnosis, taking an ACE or an ARB, left ventricular ejection fraction, left ventricular internal diastolic diameter, depression score (PHQ9) and anxiety scores (BSI Anxiety Sub-scale). Variable retained if p<0.2. LR Chi-squared=24.43, p=0.001, Pseudo R2=0.214. Abbreviations: BMI – body mass index; HF – heart failure; ACE – angiotensin converting enzyme inhibitor; ARB – angiotensin receptor blocker; PHQ9 – patient health questionnaire 9; BSI – brief symptom inventory.
Discussion
In this longitudinal analysis of physical HF symptoms and self-care behaviors over 6 month, we aimed to show how patterns of HF symptoms and self-care are associated over time as well as determine predictors of those patterns. Our study of 146 patients with symptomatic HF demonstrated that patient with more severe symptoms are significantly more engaged in self-care behaviors and that sustained high or low levels of symptom burden are associated with concordant and sustained levels of HF self-care behaviors over 6 months. Importantly, we also show clinically relevant differences in symptom groups are associated with gender, depression and an important element in the treatment of HF, prescription of an ACE inhibitor or ARB.
The results of this study support symptom severity as an important driver of engagement in HF self-care behaviors. Members of the HSS group were significantly more engaged in both self-care maintenance and self-care management behaviors at baseline and over time while members of the LSS group were less engaged in self-care. Recent research has shown better self-care associated with higher levels of symptom burden.8 It has previously been suggested that patients more bothered by symptoms may be more motivated to engage in self-care to manage current symptoms or prevent symptoms from worsening further.9
These longitudinal data provide additional information to better understand the relationship between HF self-care and symptoms. A clearer picture of how symptom and self-care are related in HF may provide new insights into how self-care influences patient outcomes such as quality of life. For example, if symptoms are motivating patients to engage more in self-care, individuals with severe symptoms but little engagement in self-care behaviors may be at increased risk for poor outcomes.27 Additionally, our results suggest patients with low symptom burden and thus potentially low engagement in self-care may benefit from further attention to achieve levels of self-care that may delay HF progression, effectively address symptoms and enhance quality of life. Further longitudinal or randomized controlled studies are needed to identify how symptoms and self-care change in relation to one another and how those relationships are associated with outcomes.
We also found women were more likely to be in the HSS group. Gender differences in physical HF symptoms have been noted in previous studies. Lee et al.28 demonstrated that gender moderated the association of physical symptoms with left ventricular diameter such that women with smaller ventricles experience worse symptom burden compared to men with smaller ventricles. Gori et al.29 found women to be more symptomatic assessed with NYHA Class compared to men. One explanation for more women being in the HSS group may be related to physiological differences in the ventricular size and function in women and men with HF.28 For instance, it has been shown that women with HF have smaller and stiffer ventricles with higher filling pressures than men, possibly contributing to more physical symptoms in women with HF.28,29 Additionally, our observation that women were significantly more likely to be in the HSS group may explain, in part, reports from other studies describing women with HF having lower health-related quality of life compared to men.30,31 Thus, our study highlights the importance of gender in HF and suggests clinicians and researchers consider gender in developing treatment plans and research projects addressing HF symptoms.
Furthermore, we also found that the HSS group which also included more women was associated with higher levels of HF self-care. Few studies have shown significant gender differences in HF self-care behaviors. Cocchieri et al.32 and Ausili et al.33 demonstrated gender was a significant predictor of self-care behaviors with women engaging more in self-care than men. It must be noted, however, these studies were based on the same data set with exclusively Italian participants. Lee and colleagues,34 on the other hand, found no clinically meaningful differences in how men and women with HF practiced self-care in a large heterogeneous multinational sample.34 This is consistent with others35,36 who also found no gender differences in the level of self-care in patients with HF. While in general, there is little data to support gender-based interventions targeting HF self-care, considering the cultural context of gender and self-care may be beneficial in developing successful interventions.37
Consistent with the literature regarding depression and symptom severity,38,39 we show higher levels of depressive symptoms associated with an increased likelihood of being a member of the HSS group. Depression and HF are thought to be reciprocal, each condition exacerbating the other.40 Both conditions share a number of pathological process such as elevated inflammatory markers and a reduction of cerebral volume that may influence the perception of symptoms.41,42 Depression has also been associated with an increase in reporting of HF symptoms.38 Additionally, depression is a known barrier to self-care behaviors contributing to poor medication adherence and decreased physical activity,6,40 potentially exacerbating physical HF symptoms. Our data supports the need to screen patients with HF for depression and address depressive symptoms to mitigate the severity of physical symptom burden and depression in HF.
Finally, we demonstrate that patients prescribed an ACE inhibitor or ARB have a significantly reduced likelihood of being in the HSS group. Our results are similar to other studies that have shown taking an ACE inhibitor/ARB is associated with lower symptom burden.43,44 Thus, the results of our study support ACE inhibitors and ARBs as important medications in the treatment of heart failure that not only improve mortality but also play an important role in reducing symptom severity. Furthermore, our study suggests that for patient who are not able to take ACE inhibitors or ARBs due to contraindications such as kidney dysfunction, low blood pressure or intolerance, alternative strategies to address symptom burden is likely to be needed. Future work should focus on reasons patient are not on guideline-directed therapies and the influence that has on patient-reported outcomes.
Strengths and Limitations
A strength of our analysis was identifying naturally-occurring symptom sub-groups with LCMA. Mixture analysis allows the examination of heterogeneity within the sample that can be masked when only evaluating sample means. Another strength was the use of latent growth modeling to examine differences in self-care at baseline values and over 6 months as a function of symptom burden. A longitudinal analysis of self-care and symptom burden can provide new insights that are not possible with cross-sectional studies. One limitation in this study is the relatively few time points. The use of three time points prevents the identification of non-linear change. Also, the selected time points may have contributed to the inability to detect change in symptoms and self-care over time. That is, changes may have occurred at time points that were not examined. Another important limitation was the use of secondary data. Variables that were not collected in the original analysis were not able to be included in this analysis. A third limitation is the small size of the HSS group may have hindered the ability of this study to detect smaller effects. Additional studies that intentionally sample patients with high and low symptom burden would facilitate a more complete understanding of differing levels of symptoms. Finally, the younger age, more prevalent non-ischemic etiology and predominantly male gender in the sample may hinder generalizability.
Conclusions
Sustained high levels of physical symptoms over 6 months were associated with persistently high levels of self-care while low symptom burden was associated with sustained lower levels of engagement in self-care. Female gender and more depressive symptoms predicted membership in the high symptoms group while being prescribed ACE inhibitors or ARBs was associated with a decreased likelihood of being in the high symptom group. Results of this study support physical symptoms as an important driver of self-care behaviors and identifies important sociodemographic and clinical factors associated with physical symptom burden that inform the care and study of patients with HF.
Supplementary Material
Implications for Practice.
In HF, high levels of self-care may indicate a high level of symptom burden.
Low levels of symptom burden could indicate low levels of self-care that may need to be addressed to optimize long-term outcomes.
Physical symptoms, particularly in women, may be improved by addressing depressive symptoms.
Patients unable to take ACE inhibitors/ARBs will likely need alternative strategies to reduce symptoms.
Acknowledgments
Funding
This work was supported by the National Institute of Nursing Research (F31NR016660) and the parent study was supported by the American Heart Association (award number 11BGIA7840062). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Nursing Research or American Heart Association.
Footnotes
Declaration of conflicting interests
The Authors declare that there is no conflict of interest
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ponikowski P, Anker SD, AlHabib KF, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1(1):4–25. doi: 10.1002/ehf2.12005. [DOI] [PubMed] [Google Scholar]
- 3.Kociol RD, McNulty SE, Hernandez AF, et al. Markers of decongestion, dyspnea relief, and clinical outcomes among patients hospitalized with acute heart failure. Circ Heart Fail. 2013;6(2):240–245. doi: 10.1161/CIRCHEARTFAILURE.112.969246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2013;128(16):240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 5.Negi S, Sawano M, Kohsaka S, et al. Prognostic Implication of Physical Signs of Congestion in Acute Heart Failure Patients and Its Association with Steady-State Biomarker Levels. PLoS ONE. 2014;9(5):e96325–e96325. doi: 10.1371/journal.pone.0096325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riegel B, Moser DK, Buck HG, et al. Self-Care for the Prevention and Management of Cardiovascular Disease and Stroke: A Scientific Statement for Healthcare Professionals From the American Heart Association. J Am Heart Assoc. 2017;6(9) doi: 10.1161/JAHA.117.006997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CS, Bidwell JT, Paturzo M, et al. Patterns of self-care and clinical events in a cohort of adults with heart failure: 1 year follow-up. Heart Lung. 2017 doi: 10.1016/j.hrtlng.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CS, Gelow JM, Mudd JO, et al. Profiles of self-care management versus consulting behaviors in adults with heart failure. European journal of cardiovascular nursing. 2013 doi: 10.1177/1474515113519188. [DOI] [PubMed] [Google Scholar]
- 9.Rockwell JM, Riegel B. Predictors of self-care in persons with heart failure. Heart & Lung. 2001;30:18–25. doi: 10.1067/mhl.2001.112503. [DOI] [PubMed] [Google Scholar]
- 10.Graven LJ, Grant JS, Gordon G. Symptomatology and Coping Resources Predict Self-Care Behaviors in Middle to Older Age Patients with Heart Failure. Nurs Res Pract. 2015;2015:840240. doi: 10.1155/2015/840240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heo S, Moser DK, Lennie TA, Fischer M, Smith E, Walsh MN. Modifiable correlates of physical symptoms and health-related quality of life in patients with heart failure: a cross-sectional study. Int J Nurs Stud. 2014;51(11):1482–1490. doi: 10.1016/j.ijnurstu.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Lee CS, Gelow JM, Denfeld QE, et al. Physical and Psychological Symptom Profiling and Event-Free Survival in Adults With Moderate to Advanced Heart Failure. The Journal of cardiovascular nursing. 2013;00(0):1–9. doi: 10.1097/JCN.0b013e318285968a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Medical A. World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 14.Jurgens CY, Lee CS, Riegel B. Psychometric Analysis of the Heart Failure Somatic Perception Scale as a Measure of Patient Symptom Perception. J Cardiovasc Nurs. 2015 doi: 10.1097/JCN.0000000000000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johns MW. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 16.Chen N, Johns MW, Li H, et al. Validation of a Chinese Version of the Epworth Sleepiness Scale. Quality of Life Research. 2002;11:817–821. doi: 10.1023/a:1020818417949. [DOI] [PubMed] [Google Scholar]
- 17.Riegel B, Lee CS, Dickson VV, Carlson B. An update on the self-care of heart failure index. The Journal of cardiovascular nursing. 2009;24(6):485–497. doi: 10.1097/JCN.0b013e3181b4baa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbaranelli C, Lee CS, Vellone E, Riegel B. Dimensionality and Reliability of the Self-Care of Heart Failure Index Scales: Further Evidence From Confirmatory Factor Analysis. Research in Nursing & Health. 2014;37(6):524–537. doi: 10.1002/nur.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riegel B, Dickson VV, Faulkner KM. The Situation-Specific Theory of Heart Failure Self-Care. The Journal of Cardiovascular Nursing. 2015;31(3):226–235. doi: 10.1097/JCN.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Willaims JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammash MH, Hall LA, Lennie TA, et al. Psychometrics of the PHQ-9 as a measure of depressive symptoms in patients with heart failure. Eur J Cardiovasc Nurs. 2013;12(5):446–453. doi: 10.1177/1474515112468068. [DOI] [PubMed] [Google Scholar]
- 22.Derogatis LR, Melisaratos N. The Brief Symptom Inventory-an introductory report-1983.pdf. 131983:595–605. [PubMed] [Google Scholar]
- 23.Khalil AA, Hall LA, Moser DK, Lennie TA, Frazier SK. The psychometric properties of the Brief Symptom Inventory depression and anxiety subscales in patients with heart failure and with or without renal dysfunction. Arch Psychiatr Nurs. 2011;25(6):419–429. doi: 10.1016/j.apnu.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Ram N, Grimm KJ. Growth Mixture Modeling: A Method for Identifying Differences in Longitudinal Change Among Unobserved Groups. Int J Behav Dev. 2009;33(6):565–576. doi: 10.1177/0165025409343765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu L, Bentler P. Fit Indices in Covariance Structure Modeling: Sensitivity to Underparameterized Model Misspecification. Psychological Methods. 1998;3(4):424–453. [Google Scholar]
- 26.MacCallum R, Browne M, Sugawara H. Power Analysis and Determination of Sample Size for Covariance Structure Modeling. Psychological Methods. 1996;1(2):130–149. [Google Scholar]
- 27.Auld J, Mudd JO, Gelow JM, Hiatt SO, Lee CS. Self-care Moderates the Relationship between Symptoms and Health-related Quality of Life in Heart Failure. Journal of Cardiovascular Nursing. 2017 doi: 10.1097/JCN.0000000000000447. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee CS, Hiatt SO, Denfeld QE, Chien CV, Mudd JO, Gelow JM. Gender-Specific Physical Symptom Biology in Heart Failure. J Cardiovasc Nurs. 2015;30(6):517–521. doi: 10.1097/JCN.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gori M, Lam CS, Gupta DK, et al. Sex-specific cardiovascular structure and function in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16(5):535–542. doi: 10.1002/ejhf.67. [DOI] [PubMed] [Google Scholar]
- 30.Riedinger MS, Dracup KA, Brecht M-L, Padilla G, Sarna L, Ganz PA. Quality of life in patients with heart failure: Do gender differences exist? Heart & Lung: The Journal of Acute and Critical Care. 2001;30(2):105–116. doi: 10.1067/mhl.2001.114140. [DOI] [PubMed] [Google Scholar]
- 31.Comin-Colet J, Anguita M, Formiga F, et al. Health-related Quality of Life of Patients With Chronic Systolic Heart Failure in Spain: Results of the VIDA-IC Study. Rev Esp Cardiol (Engl Ed) 2016;69(3):256–271. doi: 10.1016/j.rec.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 32.Cocchieri A, Riegel B, D’Agostino F, et al. Describing self-care in Italian adults with heart failure and identifying determinants of poor self-care. Eur J Cardiovasc Nurs. 2015;14(2):126–136. doi: 10.1177/1474515113518443. [DOI] [PubMed] [Google Scholar]
- 33.Ausili D, Rebora P, Di Mauro S, et al. Clinical and socio-demographic determinants of self-care behaviours in patients with heart failure and diabetes mellitus: A multicentre cross-sectional study. Int J Nurs Stud. 2016;63:18–27. doi: 10.1016/j.ijnurstu.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Lee CS, Riegel B, Driscoll A, et al. Gender differences in heart failure self-care: a multinational cross-sectional study. International journal of nursing studies. 2009;46(11):1485–1495. doi: 10.1016/j.ijnurstu.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heo S, Moser DK, Lennie TA, Riegel B, Chung ML. Gender differences in and factors related to self-care behaviors: a cross-sectional, correlational study of patients with heart failure. Int J Nurs Stud. 2008;45(12):1807–1815. doi: 10.1016/j.ijnurstu.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shuldham C, Theaker C, Jaarsma T, Cowie MR. Evaluation of the European Heart Failure Self-care Behaviour Scale in a United Kingdom population. J Adv Nurs. 2007;60(1):87–95. doi: 10.1111/j.1365-2648.2007.04371.x. [DOI] [PubMed] [Google Scholar]
- 37.Jaarsma T, Stromberg A, Ben Gal T, et al. Comparison of self-care behaviors of heart failure patients in 15 countries worldwide. Patient Educ Couns. 2013;92(1):114–120. doi: 10.1016/j.pec.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Bekelman DB, Havranek EP, Becker DM, et al. Symptoms, Depression, and Quality of Life in Patients With Heart Failure. Journal of Cardiac Failure. 2007;13(8):643–648. doi: 10.1016/j.cardfail.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Heo S, Moser DK, Pressler SJ, Dunbar SB, Dekker RL, Lennie TA. Depressive Symptoms and the Relationship of Inflammation to Physical Signs and Symptoms in Heart Failure Patients. American Journal of Critical Care. 2014;23(5):404–413. doi: 10.4037/ajcc2014614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghosh RK, Ball S, Prasad V, Gupta A. Depression in heart failure: Intricate relationship, pathophysiology and most updated evidence of interventions from recent clinical studies. Int J Cardiol. 2016;224:170–177. doi: 10.1016/j.ijcard.2016.09.063. [DOI] [PubMed] [Google Scholar]
- 41.Woo MA, Kumar R, Macey PM, Fonarow GC, Harper RM. Brain injury in autonomic, emotional, and cognitive regulatory areas in patients with heart failure. J Card Fail. 2009;15(3):214–223. doi: 10.1016/j.cardfail.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kempton MJ, Salvador Z, Munafò MR, et al. Structural neuroimaging studies in major depressive disorder: Meta-analysis and comparison with bipolar disorder. Archives of General Psychiatry. 2011;68(7):675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- 43.Lee KS, Song EK, Lennie TA, et al. Symptom Clusters in Men and Women With Heart Failure and Their Impact on Cardiac Event-Free Survival. Journal of Cardiovascular Nursing. 2010;25(4):263–272. doi: 10.1097/JCN.0b013e3181cfbb88. [DOI] [PubMed] [Google Scholar]
- 44.Group* TCTS. Effects of Enalapril on Mortality in Severe Congestive Heart Failure. New England Journal of Medicine. 1987;316(23):1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.