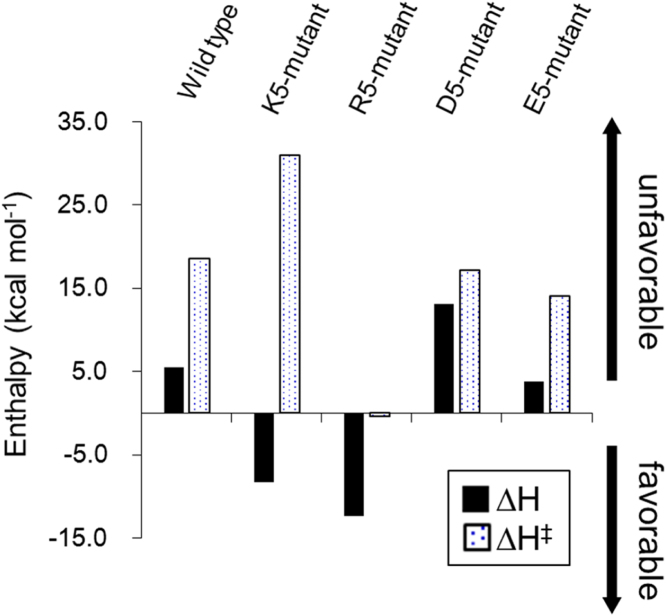
Fig. 3.
Analysis of the binding enthalpy. The activation energy parameters were obtained from the temperature dependence of the association rate constant following the Eyring approximation: ln (kon)/T) = −(∆H‡ / RT) + (∆S‡/R) + ln (kB/h) where kon is the association rate constant, ∆H‡ is the activation enthalpy, R is the gas constant, T is the absolute temperature, ∆S‡ is the activation entropy, kB is the Boltzmann constant, and h is the Planck constant.