Abstract
The carotid body (CB) is considered the main O2 chemoreceptor, which contributes to cardiorespiratory homeostasis and ventilatory acclimatization. In clinical medicine, the most common pathologies associated with the CB are tumours. However, a growing body of evidence supports the novel idea that an enhanced CB chemosensory discharge contributes to the autonomic dysfunction and pathological consequences in obstructive sleep apnoea (OSA), hypertension, systolic heart failure (HF) and cardiometabolic diseases. Heightened CB chemosensory reactivity elicited by oxidative stress has been involved in sympathetic hyperactivity, cardiorespiratory instability, hypertension and insulin resistance. CB ablation, which reduces sympathetic hyperactivity, decreases hypertension in animal models of OSA and hypertension, eliminates breathing instability and improves animal survival in HF, and restores insulin tolerance in cardiometabolic models. Thus, data obtained from preclinical studies highlight the importance of the CB in the progression of sympathetic‐related diseases, supporting the idea that appeasing the enhanced CB chemosensory drive may be useful in improving cardiovascular, respiratory and endocrine alterations. Accordingly, CB ablation has been proposed and used as a treatment for moderating resistant hypertension and HF‐induced sympathetic hyperactivity in humans. First‐in‐human studies have shown that CB ablation reduces sympathetic overactivity, transiently reduces severe hypertension and improves quality of life in HF patients. Thus, CB ablation would be a useful therapy to reverse sympathetic overactivation in HF and severe hypertension, but caution is required before it is widely used due to the crucial physiological function played by the CB. Further studies in preclinical models are required to assess side‐effects of CB ablation.
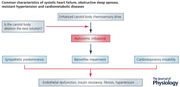
Keywords: carotid body chemoreceptor, intermittent hypoxia, hypertension, heart failure, metabolic alterations, obstructive sleep apnoea, oxidative stress, sympathetic hyperactivation
Introduction
Classical physiological functions of the carotid body
The carotid body (CB) is the principal oxygen chemoreceptor in both mammals and humans, playing crucial functions in maintaining respiratory, cardiovascular and neurohumoral homeostasis. The CBs are paired organs strategically located in the bifurcations of the common carotid arteries, weighing about 12 mg in adult health humans (Heath et al. 1970). The CB is composed of clusters of type I cells (glomus or chemoreceptor cells) organized around capillaries and surrounded by type II (sustentacular glial cells). Type I cells are the oxygen sensors that make synaptic contacts with the nerve terminals of primary sensory afferent neurons, whose perikarya are in the petrosal ganglion (Iturriaga & Alcayaga, 2004; Kumar & Prabhakar, 2012; Lopez Barneo et al. 2016). The most accepted model of O2 transduction states that hypoxia inhibits K+ channels, leading to type I cell depolarization, entry of Ca2+ through L‐type Ca2+ channels, and the release of one or more excitatory transmitters (acetylcholine and adenosine triphosphate), which in turn increases the neural discharge of the nerve endings of the chemosensory neurons. The chemotransduction process is modulated by several molecules produced within the CB, including nitric oxide (NO), hydrogen sulfide (H2S), endothelin‐1 (ET‐1) and angiotensin II (Ang II) (Iturriaga & Alcayaga, 2004; Kumar & Prabhakar, 2012). According to the classical physiological conception, the CB is crucially involved in the regulation of respiratory blood gases and pH, eliciting cardiorespiratrory and sympathetic reflexes and contributing to the ventilatory acclimatization induced by chronic hypoxaemia at high altitude. However, the CB is a complex chemoreceptor organ implicated in a series of physiological and pathological processes in health and disease. Indeed, the CB is a polymodal chemoreceptor, stimulated not only by hypoxia but also by hypercapnia, acidosis, temperature, osmolarity, and glucose. The stimulation of the CB elicits a variety of robust ventilatory, autonomic, cardiovascular, renal and endocrine responses (Kumar & Prabhakar, 2012).
A new role for the carotid body in human sympathetic‐related diseases
The CB has been implicated in some human pathologies, including sudden infant death syndrome (Gauda et al. 2004; Pozionato et al. 2013), congenital central hypoventilation syndrome (Cutz et al. 1997), and carotid body tumours. Among these pathologies, the most common clinical condition associated with the CB is the tumours, which are surgically resected or destroyed by external beam radiotherapy (Suarez et al. 2014; Fudim et al. 2015). Indeed, approximately one‐third of the total CB publications from 1900 to 2016 were connected to tumours (Fig. 1). Nevertheless, a growing body of new evidence has shown that an abnormal enhanced CB chemosensory discharge elicits sympathetic hyperreactivity, which is the common hallmark of resistant hypertension, systolic heart failure (HF), obstructive sleep apnoea (OSA) and cardiometabolic diseases (for reviews, see: Schultz et al. 2013; Iturriaga et al. 2016, 2017; Prabhakar, 2016; Conde et al. 2017). Certainly, in the last decade the idea that the CB plays a major role in the progression and maintenance of hypertension, cardiorespiratory and autonomic alterations in OSA, HF and cardiometabolic diseases has received progressive attention (Fig. 2). The results from studies using preclinical models have contributed to the novel idea that a heightened CB‐driven chemoreflex is involved in those human pathologies (Fig. 3). The augmented sympathetic activity triggered by the enhanced CB discharge, along with a reduction in the baroreflex efficiency, impairs the regulation of the vasomotor tone of blood vessels, resulting in endothelial dysfunction and hypertension. The mechanisms underlying the CB chemosensory potentiation elicited by those diseases are not completely known. However, it is likely that alteration of the normal CB chemotransduction process due to oxidative stress is responsible for the carotid chemosensory potentiation in OSA, HF and metabolic models (Peng & Prabhakar, 2003, 2004; Ding et al. 2009; Del Rio et al. 2010; Conde et al. 2017). For instance, intermittent hypoxia (IH), the main characteristic of OSA produces CB oxidative that increases ET‐1, angiotensin II and pro‐inflammatory cytokines levels in the CB (Iturriaga et al. 2014). The ablation of the CB improves animal survival in experimental models of HF (Del Rio et al. 2013a), restores autonomic balance and reduces elevated blood pressure in rats exposed to intermittent hypoxia (Del Rio et al. 2016) and spontaneous hypertensive rats (Abdala et al. 2012), and prevents insulin resistance in rats fed with high‐energy diets (Ribeiro et al. 2013; Sacramento et al. 2017). Accordingly, the surgical elimination of one or both CBs has been proposed as a clinical treatment for resistant hypertension (McBryde et al. 2013; Paton et al. 2013), and sympathetic overactivity induced by HF (Del Rio et al. 2013a; Niewinski et al. 2013; Schultz et al. 2013). Recently, two pilot clinical trial has been performed for first time in humans, to assess whether the CB ablation may ameliorate the resistant hypertension (Narkiewicz et al. 2016), and the sympathetic overactivity in HF (Niewinski et al. 2017).
Figure 1. One‐third of the total carotid body publications are connected with tumours.
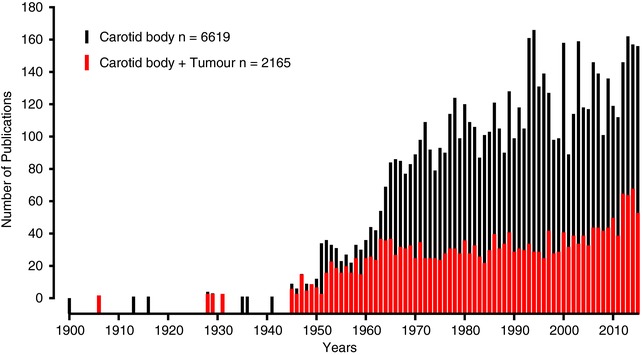
Number of publications that mention the words ‘carotid body’ plus ‘tumour and chemodectoma’ (red) related to the total number of carotid body articles (black) listed from 1900 to 2016 in PubMed (MEDLINE, US National Library of Medicine – National Institute of Health, https://www.nlm.nih.gov/)
Figure 2. Progression of the number of carotid body publications associated with sympathetic mediated diseases.
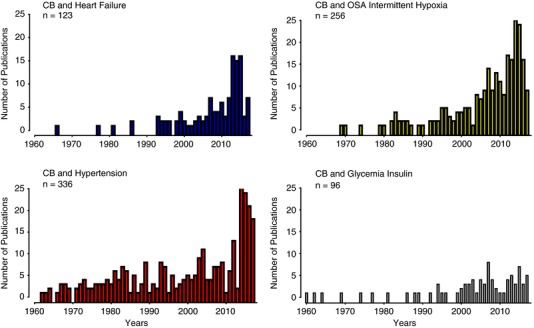
Progression of the number of publications that mention the words ‘carotid body’ plus ‘heart failure’ (blue), ‘obstructive sleep apnoea (OSA) and intermittent hypoxia’ (yellow), ‘hypertension’ (red), and ‘glycemia and insulin’ (black) listed from 1900–2016 in PubMed (MEDLINE, US National Library of Medicine – National Institute of Health, https://www.nlm.nih.gov/). Note the continuous growth in the number of articles published from 2000, showing the increasing interest in the contribution of the carotid body to the progression of cardiorespiratory and autonomic alterations in OSA, HF, hypertension and cardiometabolic diseases.
Figure 3. Contribution of the carotid body to the pathological effects mediated by sympathetic hyperactivation in human diseases.
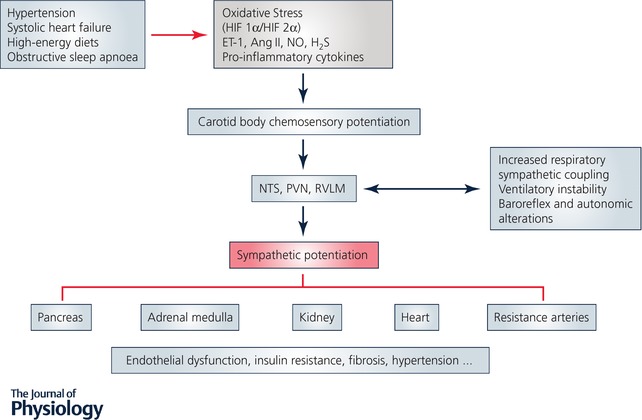
Diagram showing the pivotal role played by the carotid body in the pathological effects mediated by sympathetic hyperactivation in heart failure, obstructive sleep apnoea, hypertension and cardiometabolic diseases. Oxidative stress and inflammation are associated with carotid body chemosensory potentiation, leading to an increase in sympathetic nervous system activity, which promotes autonomic dysfunction, ventilatory instability, baroreflex alterations, hypertension, fibrosis, and insulin resistance.
Carotid body chemosensory potentiation in sympathetic‐relates diseases
Obstructive sleep apnoea
Obstructive sleep apnoea (OSA) is a growing sleep‐breathing disorder considered as an independent risk factor for systemic hypertension, and associated with stroke, heart failure, lung hypertension and atrial fibrillation (Gozal & Kheirandish‐Gozal, 2008; Somers et al. 2008; Garvey et al. 2009). During sleep, OSA patients suffer complete or partial episodes of airflow obstruction produced by the collapse of the upper airways, leading to hypoxia and hypercapnia, which stimulates the CB, eliciting ventilatory, autonomic and vasopressor reflexes (Gozal & Kheirandish‐Gozal, 2008; Somers et al. 2008; Garvey et al. 2009). The CB stimulation increases muscle respiratory activity leading to great inspiratory efforts and negative intrathoracic pressure, and finally micro‐arousals. Among these alterations, IH is considered the main factor in systemic hypertension (Gozal & Kheirandish‐Gozal, 2008; Somers et al. 2008; Garvey et al. 2009). It is well known that OSA patients show augmented cardiorespiratory and sympathetic responses to acute hypoxia, attributed to an enhanced CB chemoreflex (Narkiewicz et al. 1999; Kara et al. 2003; Somers et al. 2008). Similarly, animals exposed to IH, the gold‐standard model of OSA, develop enhanced cardiorespiratory and sympathetic responses to acute hypoxia, and hypertension (Fletcher et al. 1992; Peng & Prabhakar, 2003, 2004; Huang et al. 2009; Iturriaga et al. 2009; Del Rio et al. 2010, 2016). The autonomic imbalance produced by IH is characterized by enhanced sympathetic discharges to the kidney and muscles blood vessels, alterations of heart rate variability and reduction of the cardiac baroreflex sensitivity (Lai et al. 2006; Rey et al. 2008; Kuo et al. 2011; Prabhakar, 2016). Fletcher et al. (1992) observed that bilateral CB denervation prevented the hypertension in rats exposed to IH for 35 days, revealing a crucial role for the CB in OSA‐related hypertension. Despite this seminal observation, the idea that the CB may contribute to the cardiovascular pathologies associated with OSA was not considered until the last decade (Somers et al. 2008; Garvey et al. 2009; Iturriaga et al. 2009; Dempsey & Smith, 2014; Prabhakar 2016). New evidence has shown that an abnormal enhanced CB chemosensory discharge in both normoxia (tonic) and hypoxia (hyperreflexia) contributes to the sympathetic hyperactivity. Recordings of chemosensory discharges from the carotid sinus nerve performed by Prabhakar´s and Iturriaga´s labs have shown that IH selectively increases basal discharge in normoxia and potentiates chemosensory responses to hypoxia in rodents and cats (Peng & Prabhakar, 2003, 2004; Rey et al. 2004, 2008; Iturriaga et al. 2009; Del Rio et al. 2010). Moreover, Peng & Prabhakar (2003) showed that IH elicited long‐term facilitation of CB chemosensory discharge. They found that CB chemosensory discharges progressively increased when the CB from rats exposed to IH was excited by repetitive acute hypoxic episodes, an effect that persisted for 60 min following the end of the last hypoxic stimulus. The enhanced CB chemosensory discharge activates the NTS and the RVLM leading to sympathetic‐adrenal overflow (Peng et al. 2014; Prahabkar, 2016).
Oxidative stress enhances carotid body chemosensory discharges in intermittent hypoxia
Oxidative stress has been proposed as the key mediator of both the enhanced CB chemosensory discharge and the systemic hypertension induced by IH. Indeed, IH produces systemic and local CB oxidative stress that is involved in the enhanced chemosensory responsiveness to hypoxia (Peng & Prabhakar, 2003, 2004; Iturriaga et al. 2009; Del Rio et al. 2010). The hypertension elicited by IH in experimental models of OSA depends on the increased production of reactive oxygen (ROS) and nitrogen species (RNS), and the activation of downstream signalling pathways such as ET‐1, Ang II and pro‐inflammatory cytokines (Iturriaga et al. 2014; Prabhakar, 2016). Exposure of rats to IH increases ROS levels in the CB chemoreflex pathway including the NTS, RVLM, and adrenal medulla, an effect that is prevented by CB ablation (Peng et al. 2014). Prabhakar and colleges proposed that IH alters the balance of the hypoxia‐inducible factors HIF‐1α/HIF‐2α, favouring the transcription of pro‐oxidant enzymes leading to oxidative stress, due to the up‐regulation of pro‐oxidant enzymes (e.g. NADPH), as well as the down‐regulation of antioxidant enzymes (e.g. SOD) (for review, see Prabhakar, 2016). Rodents exposed to IH and treated with antioxidants show a reduction in systemic and local CB oxidative stress, sympathetic hyperactivation and hypertension (Peng & Prabhakar, 2003; Del Rio et al. 2010; Peng et al. 2014). The oxidative stress effects of IH are likely mediated by DNA methylation, an epigenetic mechanism. Indeed, ecitabine, a DNA hypomethylating agent, normalized ROS levels in the chemoreflex pathway (NTS, RVLM) and normalized arterial blood pressure (BP) as well as sympathetic activity (Nanduri et al. 2017). Antioxidant treatment prevents (Peng & Prabhakar, 2003; Del Rio et al. 2010) and reverses (Moya et al. 2016) the enhanced CB discharges and the systemic hypertension, making it unfeasible to establish any causal relationship between the enhanced CB discharge and the hypertension. Accordingly, to assess the causal contribution of the CB to the hypertension, we tested whether autonomic alterations, cardiac arrhythmogenesis and the hypertension induced by IH depended on the enhanced CB chemosensory drive by eliminating the CB chemosensory input (Del Rio et al. 2016). We ablated both CBs in hypertensive rats, exposed to IH (5% O2, 12 times h−1 8 h day−1) for 21 days. At day 21 of IH exposure, rats underwent bilateral CB ablation and then were exposed to IH for 7 more days. IH increased BP in 3–4 days (mean BP 10 mmHg), enhanced the ventilatory response to acute hypoxia, produced autonomic imbalance towards sympathetic predominance, reduced cardiac baroreflex gain, and increased cardiac arrhythmias. Bilateral CB ablation normalized the elevated BP, reduced the enhanced ventilatory response to hypoxia, restored cardiac autonomic and baroreflex sensitivity, and reduced the number of arrhythmias, but not the systemic oxidative stress. These results indicate that autonomic alterations induced by IH are critically dependent on CB chemosensory potentiation and support a main role for the CB in IH‐induced hypertension.
Systolic heart failure
Systolic heart failure (HF), characterized by a progressive decrease in ejection fraction is associated with autonomic imbalance, sympathetic hyperactivation and irregular breathing patterns (Sun et al. 1999; Ding et al. 2009; Schultz et al. 2013). Indeed, enhanced CB chemoreflex and sympathetic outflow have been found in HF patients and in experimental animal models (Del Rio et al. 2013a; Niewinski et al. 2013; Schultz et al. 2013). Furthermore, high hypoxic CB chemosensitivity correlates with poor prognosis and high mortality rate in patients with HF (Ponikowski et al. 2001). Schultz and colleges found an enhanced CB baseline discharge in normoxia and potentiated chemosensory responses to hypoxia in pacing‐induced HF rabbits, which in turn increased the sympathetic renal discharges (Sun et al. 1999; Ding et al. 2009). Marcus et al. (2014) found that enhanced CB chemosensory discharge increased respiratory‐sympathetic coupling in HF rabbits. They showed that the ablation of both CBs decreased pre‐sympathetic neuron activation in the RVLM, restored normal sympathetic activity, and reduced irregular breathing patterns. In addition, Del Rio et al. (2013a) found in rats with coronary artery ligation‐induced HF, a well‐established model of HF, that selective ablation of the CBs reduced pre‐sympathetic neuronal activation in the RVLM, normalized sympathetic outflow and baroreflex sensitivity, and restored breathing stability. Furthermore, if the CB ablation was performed early during the progression of HF, the manoeuvre reduced fibrosis in the ventricular myocardium, decreased the number of cardiac arrhythmias, and improved rat survival (Del Rio et al. 2013a). These results support the hypothesis that enhanced CB chemosensory discharge drives abnormal breathing patterns and increased sympathetic outflow in HF.
Oxidative stress is involved in HF‐induced carotid body potentiation
The mechanisms underlying the CB chemosensory potentiation in HF animal models is related to the diminution of CB blood flow due to the reduced cardiac output. The reduction of CB flow diminished NO production and increased oxidative stress within the CB. Li et al. (2005) demonstrated that neuronal nitric oxide synthase (nNOs) gene transfer using adenoviral vector to the CB of HF‐rabbits increased nNOS and NO levels within the CB, and most notably reversed the enhanced CB chemosensory discharges. Following reduction of arterial blood flow to the CB, NADPH oxidase was activated by Ang II due to upregulation of angiotensin converting enzyme (ACE) and downregulation of CuZn‐ and Mn‐SOD in the CB (Li et al. 2005). Schultz & Marcus (2012) proposed that the Kruppel‐like factor 2 (KLF2), a mechano‐activated transcription factor that represses ACE and induces eNOS expression in endothelial cells, is reduced in the CB. Indeed, adenoviral gene transfer of KLF2 to the CB normalizes the hypoxic ventilatory response and decreases the incidence of periodic breathing in HF rabbits (Schultz & Marcus, 2012).
Hypertension
Trzebski et al. (1982) proposed that the CB is involved in development of systemic hypertension. They found that ventilatory and BP responses to hypoxia were larger in mild‐hypertensive young subjects, compared to controls. Sinski et al. (2012) performed a randomized, crossover, placebo‐controlled study in untreated hypertensive male patients. Compared with controls, hypertensive subjects had higher resting muscle sympathetic neural activity (MSNA), and systolic and diastolic BP. Breathing 100% oxygen (Dejour´s Test), which deactivates the CB chemosensory discharge, reduced MSNA in hypertensive subjects but not in controls. Respiratory frequency, end tidal CO2 and baseline BP did not change during CB chemosensory deactivation with hyperoxia. These results suggest a tonic role for CB chemosensory drive in the development of sympathetic overactivity in hypertension. Direct evidence that CB chemosensory discharge is enhanced in hypertension was provided by Fukuda et al. (1987). They recorded CB chemosensory responses to hypoxia and hypercapnia from the carotid sinus nerve of spontaneously hypertensive rats (SHR) and found that the hypoxia chemosensitivity was higher in SHR than in control rats, while the chemosensory response to CO2 was almost the same in both groups. Abdala et al. (2012) tested the hypothesis that an enhanced CB chemosensory discharge contributes to the development and maintenance of hypertension in SHR. They cut the carotid sinus nerves in 4‐ and 12‐week‐old SHR and found that the denervation in young rats delayed the onset of hypertension, while in adult SHR the CB denervation reduced the elevated BP by ∼20 mmHg. In addition, the CB denervation decreased sympathetic hyperactivity and improved the cardiac baroreflex sensitivity (Abdala et al. 2012). It is worth noting that unilateral carotid sinus denervation was ineffective at decreasing BP, while bilateral denervation was more effective at reducing BP compared to sympathetic renal denervation (Abdala et al. 2012). These results suggest that CBs are overactive in SHR and contribute to the development and maintenance of the hypertension and the elevated sympathetic tone. More recently, Pijacka et al. (2016a) showed that carotid sinus denervation in Goldblatt hypertensive (two kidney‐one clip) rats reduced the elevated BP, which was accompanied by improvements in both baroreflex sensitivity and spectral indicators of cardiac sympatho‐vagal balance. Thus, results obtained by Paton and colleagues support the idea that peripheral CB chemoreflex should be considered as a potential therapeutic target for controlling hypertension (McBryde et al. 2013; Paton et al. 2013).
Cardiometabolic alterations
Metabolic alterations are associated with autonomic dysfunction, sympathetic hyperactivity, reduced baroreflex sensitivity and hypertension (Smith & Minson, 2012; Lehnen et al. 2013). On the other hand, the CB has been involved in the metabolic regulation of glucose and insulin (Koyama et al. 2000). Thus, it is possible that an enhanced CB chemosensory discharge may be involved in the metabolic syndrome. Accordingly, Ribeiro et al. (2013) studied the possible contribution of the CB in an experimental model of insulin resistance induced by high fat‐sucrose diets in rats. They found that CB denervation prevented the insulin resistance and hypertension induced by the high fat diet, suggesting that insulin‐induced CB chemosensory excitation contributes to increases in the sympathetic outflow, leading to a positive feedback, which results in insulin resistance and hypertension. More recently, Sacramento et al. (2017) studied whether bilateral carotid sinus neurotomy may reverse pre‐established insulin resistance, dyslipidaemia, obesity, autonomic dysfunction and hypertension in rats fed with a high‐fat diet, representing a model of insulin resistance. They found that carotid sinus neurotomy normalized sympathetic nervous system tone and reversed weight gain induced by high‐energy diets. The bilateral carotid sinus neurotomy also normalized plasma glucose and insulin levels, insulin sensitivity lipid profile, BP and endothelial function by improving glucose uptake by the liver and adipose tissue. Thus, data obtained from CB neurotomy experiments suggest an important role for the CB in metabolic alterations. However, direct neural recordings of CB chemosensory discharge in metabolically altered models are required to find out if metabolic alterations enhanced the CB chemosensory activity.
Translating carotid function into clinical medicine
New knowledge obtained from preclinical models supports CB ablation as a therapeutic tool in humans
The data obtained from preclinical models revealed the crucial role played by the CB in mediating sympathetic hyperactivation, breathing instability, autonomic alterations, insulin resistance and hypertension. The elimination of the enhanced CB chemosensory drive to the brainstem (1) reduced the elevated BP in rodent models of OSA (Del Rio et al. 2016), Goldblatt hypertension (Pijacka et al. 2016a) and spontaneous hypertension (Abdala et al. 2012); (2) reduced the breathing instability, the enhanced sympathetic activity and improved the survival rate in animal models of HF (Del Rio et al. 2013a, Marcus et al. 2014); and (3) abolished the insulin resistance in a model of rats feed with rich‐energy diets (Ribeiro et al. 2013; Sacramento et al. 2017). Taken together, these data strongly suggest that an enhanced CB chemosensory discharge contributes to potentiate the sympathetic activity leading to the development and progression of cardiorespiratory and metabolic diseases in humans. According, Paton and colleagues proposed the ablation of the CB as an anti‐hypertensive treatment in drug‐resistant hypertensive and HF patients (McBryde et al. 2013; Narkiewicz et al. 2016; Paton et al. 2013).
Pilot CB ablation studies in humans with persistent hypertension or heart failure
Recently, results from two safety pilot studies that examined the feasibility and potential benefits of he CB ablation in humans suffering from severe hypertension and HR have been reported (Narkiewicz et al. 2016; Niewinsky et al. 2017). Narkiewicz et al. (2016) performed a pilot study to assess the effectiveness, safety and feasibility of CB ablation in patients with hypertension resistant to pharmacological treatment. This work was the first‐in‐man study to assess the effects of unilateral CB resection in 7 male and 8 female resistant hypertensive patients with systolic and diastolic pressures over 180 and 103 mmHg, respectively. When BP data were pooled from all the patients, no statistical differences were found following the unilateral CB resection. According to the observed effects on BP, the authors classified patients into two groups: (1) responders – 8 patients with evidence of glomus cells in the resected tissue and a ≥10 mmHg drop in BP at 3 month follow‐up visit; (2) non‐responders – 6 patients with evidence of glomus cells in the resected tissue, but who did not show a ≥10 mmHg drop in BP after 3 month follow‐up. At 3 months after the CB resection, responders showed a reduced daytime BP that persisted 6 months after resection, but not at 12 months. Nighttime BP showed similar behaviour. It is worth noting that the BP level in the responder group remained lower compared to the non‐responder group at 12 months. The responder group showed a higher ventilatory response to hypoxia, suggesting an enhanced CB chemoreflex. In addition,the responders group showed a reduction in MSNA with a similar time course to the BP drop, suggesting a reduction in vasomotor tone. Narkiewicz et al. (2016) also found an improvement in baroreflex sensitivity in the responder group, but not in the non‐responder patients, which contributed to lowering BP. Thus, it is plausible that unilateral CB ablation may be useful in reducing elevated BP in a subset of severe hypertensive patients with enhanced ventilatory chemoreflex responses. Fudim et al. (2015) also found that unilateral resection of a CB tumour in one patient with hypertension failed to decrease BP. The transient reduction in the elevated BP following unilateral CB resection suggested that the enhanced chemosensory discharge in the remained CB was enough to elevate BP in the long term. Classical experiments involving sinoaortic denervation in animals have shown that peripheral baroreceptors and chemoreceptors do not contribute to setting the long‐term BP level, but contribute to attenuating short‐term BP variability. The long‐term BP set‐point is determined by the kidney, which controls osmolarity and corporal volume (Cowley et al. 1973).
Niewinsky et al. (2017) reported the effects CB resection on sympathetic hyperactivity in human systolic HF. This first‐in‐man study was performed in 10 male patients with systolic HF with a left ventricle ejection of 27 ± 7%. From the 10 patients, 6 patients underwent unilateral right‐sided CB ablation and the remaining 4 bilateral CB ablation. Patients showed a reduced ventilatory response to hypoxia and MNSA levels during 2 months after the CB ablation, and some improved exercise tolerance. Their quality of life showed a significant improvement at 1 month, but the score measured at 2 months did not show significant differences. Fatigue and shortness of breath scores showed some improvement, but only the fatigue score was statistically different at 1 and 2 months. The authors observed a trend towards worsening nocturnal O2 saturation at night in patients with bilateral CB resection, while one case required non‐invasive ventilation. They concluded that this first study in man showed that CBs resection in patients with systolic HF is associated with a decrease in sympathetic activity, although bilateral CB ablation may produce a risk of worsening nocturnal oxygenation.
Perspectives
New evidence obtained by several research groups has contributed to the novel idea that an enhanced CB chemosensory discharge triggers sympathetic hyperactivity in human pathologies. This new knowledge supported the idea that the reduction or elimination of the heightened CB tonic chemosensory drive and potentiated hypoxic responses could be used as a therapeutic approach to reduce sympathetic hyperactivation, as well as severe hypertension, in humans. Furthermore, the results of recent pilot studies performed in patients show the feasibility and safety of this approach (Narkiewicz et al. 2016; Niewinski et al. 2017). Accordingly, a clinical trial is on course to assess the feasibility of unilateral endovascular CB ablation in a large cohort of patients with refractory hypertension (http://clinicaltrials.gov:NCT02519868).
Since, the CB plays a crucial role during hypoxia, exercise and sleep, some concerns about the potential side‐effects of CB ablation have been raised, which may preclude wide use of this manoeuvre (Dempsey & Smith, 2014: Johnson & Joyner, 2013). The main concerns are:
-
(1)
Lack of hypoxic responses following CBs ablation, which may result in nocturnal oxygen desaturation. Nakayama (1961) reported that bilateral CB ablation performed in asthma patients to improve the sensation of air hunger resulted in reduction of BP. However, Honda (1985) studied some of these patients 20 years after the CB resection and found a 90% loss of ventilatory reflex response to hypoxia and a depression of CO2 chemosensitivity. Similarly, Wade et al. (1970) studied the ventilatory responses to hypoxia and hypercapnia in patients before and 1–9 weeks after CB endarterectomy for transient attacks of cerebral ischaemia. They found that the ventilatory responses remained unchanged in patients that underwent unilateral CB endarterectomy, while the hypoxic response was abolished in patients following bilateral CB endarterectomy. Thus, bilateral CB endarterectomy resulted in a loss of CB function. The blunted hypoxic response following bilateral CB ablation may preclude exposure to hypoxia at high altitude and in airplanes. The cabin pressurization in modern airplanes is equivalent to an altitude of 2100–2400 m, which is safe for normal subjects, but perhaps not for CB‐ablated patients with blunted respiratory and sympathetic responses to hypoxia.
-
(2)
Selectivity of the CB ablation without affecting baroreceptor function. Some studies that assessed baroreceptor function showed an increased BP variability and a reduced baroreflex sensitivity following bilateral CB resection (Fudim et al. 2015).
-
(3)
Associated comorbidities. Many HF and hypertensive patients may develop during their lives OSA and/or metabolic alterations (hyperglycaemia, dyslipidaemia, insulin resistance), with the chance of suffering apnoea episodes and severe nocturnal hypoxaemias. Thus, the utilization of drugs that reduce enhanced CB chemosensory discharge is an attractive possibility. Indeed, Pijacka et al. (2016b) found that antagonism of P2X3 receptors reduced BP, basal sympathetic activity and normalized enhanced CB hyperreflexia in conscious rats with hypertension, while no effect was observed in rats without hypertension. On the other hand, Del Rio et al. (2013b) found that H2S inhibition in rats with congestive HF reduced the apnoea index by 90%, the breathing variability by 40–60%, and reversed the enhanced CB chemosensory discharges and chemoreflex responses Taken together, these results support the idea that pharmacological treatments could be used to decrease the enhanced CB chemosensory drive, sympathetic hyperactivation outflow and cardiorespiratory alterations.
Summary
In conclusion, CB ablation could be a useful method to reverse enhanced CB chemosensory discharges and chemoreflexes in HF and severe hypertension, but caution is required before widespread clinical use of bilateral CB ablation or bilateral carotid sinus neurotomy, which abolished ventilatory responses to hypoxia and may eliminate baroreceptor fibres. Certainly, new preclinical studies are needed to assess the long‐term effects of CB ablation on pathological consequences and mortality rates in experimental models of obstructive sleep apnoea and cardiometabolic syndrome. The use of new models mimicking human comorbidities (OSA, HF, metabolic syndrome, ageing) will be helpful to evaluate side‐effects of CB ablation.
Additional information
Competing interests
The author declares no conflicts of interest relevant to this manuscript.
Funding
This work was supported by grant 1150040 from the National Fund for Scientific and Technological Development of Chile (FONDECYT).
Biography
Rodrigo Iturriaga's research focuses on understanding the contribution of the carotid body to the autonomic and cardiorespiratory alterations induced by intermittent hypoxia, the main characteristic of obstructive sleep apnoea. He has been interested in the contribution of transmitters (ATP and ACh), modulators (ROS, NO, CO, and ET‐1) and carbonic anhydrase‐CO2‐HCO3 − in the carotid body chemosensory process. Rodrigo Iturriaga received a BSc in Biology from the Universidad de Concepcion, Chile, and his PhD in Physiology from the Pontificia Universidad Católica de Chile under the advice of Dr Patricio Zapata. He did his postdoctoral studies with Dr Sukhamay Lahiri at the Department of Physiology, University of Pennsylvania, USA. Presently, he is Full Professor and Chairman of the Department of Physiology at the Faculty of Biological Sciences, Pontificia Universidad Católica de Chile. He is a member of several scientific societies including the American Physiological Society, the Society for Neurocience, Sociedad Española de Ciencias Fisiológicas and Sociedad Chilena de Ciencias Fisiológicas.

Edited by: Harold Schultz & Silvia Conde
This review was presented at the symposium ‘Advances in cellular and integrative control of oxygen and carbon dioxide homeostasis’, which took place at the XX ISAC meeting, Baltimore, MD, USA, 23–27 July 2017.
References
- Abdala AP, McBryde FD, Marina N, Hendy EB, Engel man ZJ, Fudim M, Sobotka PA, Gourine AV & Paton JFR (2012). Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J Physiol 590, 4269–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde SV, Ribeiro MJ, Melo BF, Guarino MP & Sacramento JF (2017). Insulin resistance: a new consequence of altered carotid body chemoreflex? J Physiol 595, 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley AW, Liard JF & Guyton AC (1973). Role of the baroreceptor reflex in daily control of arterial blood pressure and other variables in dogs. Circ Res 32, 564–576. [DOI] [PubMed] [Google Scholar]
- Cutz E, Ma TK, Perrin DG, Moore AM & Becker LE (1997). Peripheral chemoreceptors in congenital central hypoventilation syndrome. Am J Respir Crit Care Med 155, 358–363. [DOI] [PubMed] [Google Scholar]
- Del Rio R, Moya EA & Iturriaga R (2010). Carotid body and cardiorespiratory alterations in intermittent hypoxia: the oxidative link. Eur Respir J 36, 143–150 [DOI] [PubMed] [Google Scholar]
- Del Rio R, Marcus NJ & Schultz HD (2013a). Carotid chemoreceptor ablation improves survival in heart failure: rescuing autonomic control of cardiorespiratory function. J Am Coll Cardiol 62, 2422–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio R, Marcus NJ & Schultz HD (2013b). Inhibition of hydrogen sulfide restores normal breathing stability and improves autonomic control during experimental heart failure. J Appl Physiol 114, 1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio R, Andrade DC, Lucero C, Arias P & Iturriaga R (2016). Carotid body ablation abrogates hypertension and autonomic alterations induced by intermittent hypoxia in rats. Hypertension 68, 436–445. [DOI] [PubMed] [Google Scholar]
- Dempsey JA & Smith CA (2014). Pathophysiology of human ventilatory control. Eur Respir J 44, 495–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Li YL, Zimmerman MC, Davisson RL & Schultz HD (2009). Role of CuZn superoxide dismutase on carotid body function in heart failure rabbits. Cardiovasc Res 81, 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher EC, Lesske J, Behm R, Miller CC, Stauss H & Unger T (1992). Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol 72, 1978–1984. [DOI] [PubMed] [Google Scholar]
- Fudim M, Groom KL, Laffer CL, Netterville JL, Robertson D & Elijovich F (2015). Effects of carotid body tumor resection on the blood pressure of essential hypertensive patients. J Am Soc Hypertens 9, 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y, Sato A & Trzebski A (1987). Carotid chemoreceptor discharge responses to hypoxia and hypercapnia in normotensive and spontaneously hypertensive rats. J Auton Nerv Syst 19, 1–11. [DOI] [PubMed] [Google Scholar]
- Garvey JF, Taylor CT & McNicholas WT (2009). Cardiovascular disease in obstructive sleep apnoea syndrome: the role of intermittent hypoxia and inflammation. Eur Respir J 33, 1195–1205. [DOI] [PubMed] [Google Scholar]
- Gauda EB, McLemore GL, Tolosa J, Marston‐Nelson J & Kwak D (2004). Maturation of peripheral arterial chemoreceptors in relation to neonatal apnoea. Semin Neonatol 9, 181–194. [DOI] [PubMed] [Google Scholar]
- Gozal D & Kheirandish‐Gozal L (2008). Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med 177, 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath D, Edwards C & Harris P (1970). Post‐mortem size and structure of the human carotid body. Thorax 25, 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y ( 1985). Role of carotid chemoreceptors in control of breathing at rest and in exercise: studies of human subjects with bilateral carotid body resection. Jap J Physiol 35, 535–544. [DOI] [PubMed] [Google Scholar]
- Huang J, Lusina S, Xie T, Ji E, Xiang S, Liu Y & Weiss JW (2009). Sympathetic response to chemostimulation in conscious rats exposed to chronic intermittent hypoxia. Respir Physiol Neurobiol 166, 102–106. [DOI] [PubMed] [Google Scholar]
- Iturriaga R & Alcayaga J (2004). Neurotransmission in the carotid body: transmitters and modulators between glomus cells and petrosal ganglion nerve terminals. Brain Res Rev 47, 46–53. [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Andrade DC & Del Rio R (2014). Enhanced carotid body chemosensory activity and the cardiovascular alterations induced by intermittent hypoxia. Front Physiol 5, 468–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriaga R, Del Rio R, Idiaquez J & Somers VK (2016). Carotid body chemoreceptors, sympathetic neural activation, and cardiometabolic disease. Biol Res 49, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriaga R, Moya EA & Del Rio R (2009). Carotid body potentiation induced by intermittent hypoxia: implications for cardiorespiratory changes induced by sleep apnoea. Clin Exp Pharmacol Physiol 36, 1197–1204. [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Oyarce MP & Dias ACR (2017). Role of carotid body in intermittent hypoxia‐related hypertension. Curr Hypertens Rep 19, 38. [DOI] [PubMed] [Google Scholar]
- Johnson BD & Joyner MJ (2013). Carotid body denervation. Too soon to get breathless about heart failure. J Am Coll Cardiol 62, 2431–2432. [DOI] [PubMed] [Google Scholar]
- Kara T, Narkiewicz K & Somers VK (2003). Chemoreflexes – physiology and clinical implications. Acta Physiol Scand 177, 377–384. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Coker RH, Stone EE, Lacy DB, Jabbour K, Williams PE & Wasserman DH (2000). Evidence that carotid bodies play an important role in glucoregulation in vivo. Diabetes 49, 1434–1442. [DOI] [PubMed] [Google Scholar]
- Kumar P & Prabhakar NR (2012). Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol 2, 141–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo TB, Yuan ZF, Lin YS, Lin YN, Li WS, Yang CC & Lai CJ (2011). Reactive oxygen species are the cause of the enhanced cardiorespiratory response induced by intermittent hypoxia in conscious rats. Respir Physiol Neurobiol 170, 70–79. [DOI] [PubMed] [Google Scholar]
- Lai CJ, Yang CC, Hsu YY, Lin YN & Kuo TB (2006). Enhanced sympathetic outflow and decreased baroreflex sensitivity are associated with intermittent hypoxia‐induced systemic hypertension in conscious rats. J Appl Physio 100, 1974–1982. [DOI] [PubMed] [Google Scholar]
- Lehnen AM, Leguisamo NM, Casali KR & Schaan BD (2013). Progressive cardiovascular autonomic dysfunction in rats with evolving metabolic syndrome. Auton Neurosci 176, 64–69. [DOI] [PubMed] [Google Scholar]
- Li YF, Liu D, Cornish KG, Patel KP, Zucker IH, Channon KM & Schultz HD (2005). Gene transfer of neuronal nitric oxide synthase to carotid body reverses enhanced chemoreceptor function in heart failure rabbits. Circ Res 97, 260–267. [DOI] [PubMed] [Google Scholar]
- López‐Barneo J, Ortega‐Sáenz P, González‐Rodríguez P, Fernández‐Agüera MC, Macías D, Pardal R & Gao L (2016). Oxygen‐sensing by arterial chemoreceptors: Mechanisms and medical translation. Moll Aspects Med 47‐48, 90–108. [DOI] [PubMed] [Google Scholar]
- McBryde FD, Abdala AP, Hendy EB, Pijacka W, Marvar P, Moraes DJ, Sobotka PA & Paton JFR (2013). The carotid body as a putative therapeutic target for the treatment of neurogenic hypertension. Nat Commun 4, 2395. [DOI] [PubMed] [Google Scholar]
- Marcus NJ, Del Rio R, Schultz EP, Xia XH & Schultz HD (2014). Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J Physiol 592, 391–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya EA, Arias P, Varela C, Oyarce MP, Del Rio R & Iturriaga R (2016). Intermittent hypoxia‐induced chemosensory potentiation and hypertension are critically dependent on peroxynitrite formation. Oxid Med Cell Long 2016, 9802136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K (1961). Surgical removal of the carotid body for bronchial asthma. Dis Chest 40, 595–604. [DOI] [PubMed] [Google Scholar]
- Nanduri J, Peng YJ, Wang N, Khan SA, Semenza GL, Kumar GK & Prabhakar NR (2017). Epigenetic regulation of redox state mediates persistent cardiorespiratory abnormalities after long‐term intermittent hypoxia. J Physiol 595, 63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkiewicz K, Ratcliffe LEK, Hart EC, Brian LJB, Chrostowska M, Wolf J, Szyndler A, Hering D, Abdala AP, Manghat N, Burchell AE, Durant C, Lobo MD, Sobotka PA, Patel NK, Leiter JC, Engelman ZJ, Nightingale AK & Paton JRF (2016). Unilateral carotid body resection in resistant hypertension: a safety and feasibility trial JACC Basic Transl . Sci 1, 313–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkiewicz K, van de Borne PJ, Penske CA, Dyken ME, Montano N, Somers VK (1999). Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation 99, 1183–1189. [DOI] [PubMed] [Google Scholar]
- Niewinski P, Janczak D, Rucinski A, Jazwiec P, Sobotka PA, Engelman ZJ, Fudim M, Tubek S, Jankowska EA, Banasiak W, Hart EC, Paton JRF & Ponikowski P (2013). Carotid body removal for treatment of chronic systolic heart failure. Int J Cardiol 168, 2506–2509. [DOI] [PubMed] [Google Scholar]
- Niewinski P, Janczak D, Rucinski A, Tubek S, Engelman ZJ, Piesiak P, Jazwiec P, Banasiak W, Fudim M, Sobotka PA, Javaheri S, Hart EC, Paton JFF & Ponikowski P (2017). Carotid body resection for sympathetic modulation in systolic heart failure: results from first‐in‐man study. Eur J Heart Failure 19, 391–400. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Sobotka PA, Fudim M, Engleman ZJ, Hart EC, McBryde FD, Abdala AP, Marina N, Gourine AV, Lobo M, Patel N, Burchell A, Ratcliffe L & Nightingale A (2013). The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension 61, 5–13. [DOI] [PubMed] [Google Scholar]
- Peng YJ & Prabhakar NR (2003). Reactive oxygen species in the plasticity of breathing elicited by chronic intermittent hypoxia. J Appl Physiol 94, 2342–2349. [DOI] [PubMed] [Google Scholar]
- Peng Y & Prabhakar NR (2004). Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol 96, 1236–1242. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Yuan G, Khan S, Nanduri J, Makarenko VV, Reddy VD, Vasavda C, Kumar GK, Semenza GL & Prabhakar NR (2014) Regulation of hypoxia‐inducible factor‐α isoforms and redox state by carotid body neural activity in rats. J Physiol 592, 3841–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijacka W, McBryde FD, Marvar P, Lincevicius G, Abdala AP, Woodward L, Li D, Paterson DJ & Paton JFR (2016a), Carotid sinus denervation ameliorates renovascular hypertension in adult Wistar rats. J Physiol 594, 6255–6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijacka W, Moraes DJ, Ratcliffe LEK, Nightingale AK, Hart ECJ, Silva Md, Machado B, McBryde FD, Sheikh APA, Ford A & Paton JFR (2016b). Purinergic receptors in the carotid body as a new drug target for controlling hypertension. Nat Medicine 22, 1151–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponikowski P, Chua TP, Anker SD, Francis DP, Doehner Banasiak W, Poole‐Wilson PA, Piepoli MF & Coats AJ (2001). Peripheral chemoreceptor hypersensitivity: an ominous sign in patients with chronic heart failure. Circulation 104, 544–549. [DOI] [PubMed] [Google Scholar]
- Pozionato A, Macchi V, Stecco C & De Caro R (2013). The carotid body in sudden infant death syndrome. Respir Physiol Neurobiol 185, 194–201. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR (2016). Carotid body chemoreflex: a driver of autonomic abnormalities in sleep apnoea. Exp Physiol 101, 975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey S, Del Rio R, Alcayaga J & Iturriaga R (2004). Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J Physiol 560, 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey S, Tarvainen MP, Karjalainen PA & Iturriaga R (2008). Dynamic time‐varying analysis of heart rate and blood pressure variability in cats exposed to short‐term chronic intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol 295, R28–R37. [DOI] [PubMed] [Google Scholar]
- Ribeiro MJ, Sacramento JF, Gonzalez C, Guarino MP, Monteiro EC & Conde SV (2013). Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes 62, 2905–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacramento JF, Ribeiro MJ, Rodrigues T, Olea E, Melo BF, Guarino MP, Fonseca‐Pinto R, Ferreira CR, Coelho J, Obeso A, Seiça R, Matafome P & Conde SV (2017). Functional abolition of carotid body activity restores insulin action and glucose homeostasis in rats: key roles for visceral adipose tissue and the liver. Diabetologia 60, 158–168. [DOI] [PubMed] [Google Scholar]
- Sinski M, Lewandowski J, Przybylski J, Bidiuk J, Abramczyk P, Ciarka A & Gaciong Z (2012). Tonic activity of carotid body chemoreceptors contributes to the increased sympathetic drive in essential hypertension. Hypertens Res 35, 487–491. [DOI] [PubMed] [Google Scholar]
- Smith M & Minson CT (2012). Obesity and adipokines: effects on sympathetic overactivity. J Physiol 590, 1787–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M & Young T (2008). Sleep apnea and cardiovascular disease: An American Heart Association. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research NIH. J Am Coll Cardiol 52, 686–717. [DOI] [PubMed] [Google Scholar]
- Schultz HD & Marcus NJ (2012). Heart failure and carotid body chemoreception. Adv Exp Med Biol 758, 387–395. [DOI] [PubMed] [Google Scholar]
- Schultz HD, Marcus NJ & Del Rio R (2013). Role of the carotid body in the pathophysiology of heart failure. Curr Hypertens Rep 15, 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez C, Rodrigo JP, Mendenhall WM, Hamoir M, Silver CE, Grigoriev V, Struan P, Neumann HPH, Obholzer R, Offergeld C & Langendijk JA (2014). Carotid body paragangliomas: a systematic study on management with surgery and radiotherapy. Eur Arch Otorhinolaryngol 27, 23–34. [DOI] [PubMed] [Google Scholar]
- Sun S, Wang W, Zucker IH & Schultz HD (1999). Enhanced peripheral chemoreflex function in conscious rabbits with pacing‐induced heart failure. J Appl Physiol 86, 1264–1272. [DOI] [PubMed] [Google Scholar]
- Trzebski A, Tafil M, Zoltowski M & Przybylski J (1982). Increased sensitivity of the arterial chemoreceptor drive in young men with mild hypertension. Cardiovasc Res 16, 163–172. [DOI] [PubMed] [Google Scholar]
- Wade JG, Larson CP Jr, Hickey RF, Ehrenfeld WK & Severinghaus JW (1970). Effect of carotid endarterectomy on carotid chemoreceptor and baroreceptor function in man. N Engl J Med 282, 823–829. [DOI] [PubMed] [Google Scholar]


