Abstract
Key points
Carotid bodies play a critical role in maintaining arterial pressure during hypoxia and this has important implications when considering resection therapy of the carotid body in disease states such as hypertension.
Curbing hypertension in patients whether resting or under stress remains a major global health challenge.
We demonstrated previously the benefits of removing carotid body afferent input into the brain for both alleviating sympathetic overdrive and reducing blood pressure in neurogenic hypertension.
We describe a new approach in rats for selective ablation of the carotid bodies that spares the functional integrity of the carotid sinus baroreceptors, and demonstrate the importance of the carotid bodies in the haemodynamic response to forced exercise, hypoxia and hypercapnia in conditions of hypertension.
Selective ablation reduced blood pressure in hypertensive rats and re‐set baroreceptor reflex function accordingly; the increases in blood pressure seen during exercise, hypoxia and hypercapnia were unaffected, abolished and augmented, respectively, after selective carotid body removal.
The data suggest that carotid body ablation may trigger potential cardiovascular risks particularly during hypoxia and hypercapnia and that suppression rather than obliteration of their activity may be a more effective and safer route to pursue.
Abstract
The carotid body has recently emerged as a promising therapeutic target for treating cardiovascular disease, but the potential impact of carotid body removal on the dynamic cardiovascular responses to acute stressors such as exercise, hypoxia and hypercapnia in hypertension is an important safety consideration that has not been studied. We first validated a novel surgical approach to selectively resect the carotid bodies bilaterally (CBR) sparing the carotid sinus baroreflex. Second, we evaluated the impact of CBR on the cardiovascular responses to exercise, hypoxia and hypercapnia in conscious, chronically instrumented spontaneously hypertensive (SH) rats. The results confirm that our CBR technique successfully and selectively abolished the chemoreflex, whilst preserving carotid baroreflex function. CBR produced a sustained fall in arterial pressure in the SH rat of ∼20 mmHg that persisted across both dark and light phases (P < 0.001), with baroreflex function curves resetting around lower arterial pressure levels. The cardiovascular and respiratory responses to moderate forced exercise were similar between CBR and Sham rats. In contrast, CBR abolished the pressor response to hypoxia seen in Sham animals, although the increases in heart rate and respiration were similar between Sham and CBR groups. Both the pressor and the respiratory responses to 7% hypercapnia were augmented after CBR (P < 0.05) compared to sham. Our finding that the carotid bodies play a critical role in maintaining arterial pressure during hypoxia has important implications when considering resection therapy of the carotid body in disease states such as hypertension as well as heart failure with sleep apnoea.
Keywords: peripheral chemoreceptor, selective ablation, blood pressure, exercise, baroreflex
Key points
Carotid bodies play a critical role in maintaining arterial pressure during hypoxia and this has important implications when considering resection therapy of the carotid body in disease states such as hypertension.
Curbing hypertension in patients whether resting or under stress remains a major global health challenge.
We demonstrated previously the benefits of removing carotid body afferent input into the brain for both alleviating sympathetic overdrive and reducing blood pressure in neurogenic hypertension.
We describe a new approach in rats for selective ablation of the carotid bodies that spares the functional integrity of the carotid sinus baroreceptors, and demonstrate the importance of the carotid bodies in the haemodynamic response to forced exercise, hypoxia and hypercapnia in conditions of hypertension.
Selective ablation reduced blood pressure in hypertensive rats and re‐set baroreceptor reflex function accordingly; the increases in blood pressure seen during exercise, hypoxia and hypercapnia were unaffected, abolished and augmented, respectively, after selective carotid body removal.
The data suggest that carotid body ablation may trigger potential cardiovascular risks particularly during hypoxia and hypercapnia and that suppression rather than obliteration of their activity may be a more effective and safer route to pursue.
Introduction
The carotid bodies have recently emerged as a promising therapeutic target for treating hypertension (Paton et al. 2013; Ratcliffe et al. 2014; Narkiewicz et al. 2016; Pijacka et al. 2016) and other cardiovascular diseases such as heart failure (Schultz & Marcus, 2012; Niewinski et al. 2013, 2017; Andrade et al. 2015), where the peripheral chemoreceptors exhibit increases in both sensitivity and tonicity (Abdala et al. 2012; McBryde et al. 2013; Pijacka et al. 2016). We, and others, suggest that abnormal chemoreflex activity drives a long‐term increase in sympathetic over‐activity, resulting in a chronic, neutrally mediated hypertension (Sinski et al. 2012; McBryde et al. 2013; Moraes et al. 2015; Pijacka et al. 2016). However, the role that the carotid bodies play in mediating the dynamic cardiovascular response to acute stressors such as exercise, hypoxia and hypercapnia has not previously been studied under conditions of hypertension and may have important clinical implications especially if the carotid bodies are targeted surgically.
Exercise presents a major challenge to the cardiovascular system, where a pronounced functional hyperaemia in skeletal muscle vasculature, and subsequent fall in total peripheral resistance (TPR), must be countered by an opposing sympathetically mediated vasoconstriction to maintain or increase arterial pressure in order to avoid compromising organ perfusion (Mitchell et al. 1983). The pressor response to exercise is mediated by activation of the sympathetic nervous system, and has been shown to be augmented in hypertension (Smith et al. 2006; Delaney et al. 2010). However, in hypertensive patients, exercise tolerance has been shown to be reduced by up to 30% vs. age‐matched normotensive patients (Lim et al. 1996); this may be a consequence of poor skeletal muscle blood flow due to intense vasoconstriction and/or reduced sympatholysis mediated by release of local metabolites. With the evidence that carotid bodies become more active in exercise (Linton & Band, 1985; Jacobi et al. 1989; Ward, 1994; Paterson, 1996; Chu et al. 2007), they may play an essential role in ensuring that arterial pressure is maintained. Given their tonicity and sensitisation in hypertension (see above) the carotid body reflex vasoconstrictor response may be exaggerated during exercise and offset sympatholysis. We have directly addressed this idea.
The classical stimulant for the carotid bodies is hypoxia. However, hypoxia also reduces vascular smooth muscle tone, causing a lowering of TPR and reduction in arterial pressure (Kulandavelu et al. 2015). The hypoxia‐induced reduction in TPR may be opposed by homeostatic‐reflexes that increase sympathetic outflow to maintain arterial pressure and preserve organ blood flow; the peripheral chemoreceptors participate in such mediation (Marshall & Metcalfe, 1988; Itskovitz et al. 1991; Stein et al. 1999). Importantly, if the carotid bodies are to be a clinically viable treatment target for cardiovascular disease, an understanding of the ability of the cardiovascular system to cope with hypoxia is highly pertinent, especially given the high prevalence of sleep apnoea in patients with cardiovascular diseases.
Hypoxia alone rarely occurs without concomitant hypercapnia, which also stimulates peripheral chemoreceptors (Pepper et al. 1996; Vidruk et al. 2001). However, hypercapnia also stimulates central chemoreceptors to drive hyperventilation, increased blood pressure and elevated sympathetic activity (Kanbar et al. 2010; Takakura & Moreira, 2011). To study the effect of hypercapnia on the carotid body without co‐activation of central chemoreceptors, previous studies have either isolated the carotid body circulation from the cerebral circulation or assessed responses before and after carotid body denervation. The evidence suggests that hypercapnic stimulation of the carotid bodies in rats triggers hyperpnoea (Fiamma et al. 2013), but the sympathoexcitatory and pressor responses evoked by hypercapnia remained unchanged after denervation of carotid bodies in conscious rats, suggesting they play little role in mediating these cardiovascular responses (Oikawa et al. 2005; Sabino et al. 2013). We have re‐assessed this here to ensure that appropriate cardiovascular adjustment can be made during hypercapnia after selective carotid body resection.
Based on the cited studies described above, we tested the hypothesis that selective carotid body resection in hypertensive rats would lead to an inability to maintain control of arterial pressure during the stressors of both exercise and hypoxia but not hypercapnia.
Methods
Ethical approval
All procedures were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986, under licence to the Home Office. All investigators understand the ethical principles under which the Journal of Physiology operates, and their work complies with the animal ethics checklist described by Grundy (2015). Experiments were conducted on 16–20 week old male spontaneously hypertensive (SH) rats bred within the University of Bristol Animal Services Unit and housed with a 12/12 h light (07.00–18.00 h)/dark (19.00–06.00 h) period and ad libitum access to food and water. In total 29 rats were used.
Validation of selective carotid body resection in the terminally anaesthetised SH rat
These experiments were performed to establish and validate our selective carotid body removal procedure. Rats (n = 11) were anaesthetised with intramuscular injections of ketamine (60 mg kg−1; Vetalar, Zoetis, London, UK) and medetomidine hydrochloride (250 μg kg−1; Domitor, Elanco Animal Health, Basingstoke, UK). Anaesthetic depth was assessed by the withdrawal reflex following a pinch to the tail or a hind paw and an additional 1/6 of an initial dose of ketamine/medetomidine hydrochloride given as needed. The femoral vein was catheterised to allow venous access for drug infusions, and arterial pressure was measured via radio‐telemetry (see below). Through a midline neck incision, the common carotid arteries were cleared, and 3.0 silk suture was used to allow easy retraction and improve access to the carotid bifurcation. In all animals the aortic depressor nerves were identified, as described previously (Pickering et al. 2008) and sectioned bilaterally (ADNX). Following a 30 min recovery period to record a stable baseline, the chemoreflex was tested with an i.v. bolus infusion of sodium cyanide (100 ul; 0.04%), and baroreflex function assessed using i.v. infusions of vasoactive drugs phenylephrine (0.1 mg ml−1, i.v.) and sodium nitroprusside (0.1 mg ml−1, i.v.) (Sigma‐Aldrich Co., Poole, UK) to produce ramp changes in arterial pressure, as described previously (Abdala et al. 2012; McBryde et al. 2013; Lincevicius et al. 2015). Animals were then randomly divided into two groups – Time Control and Carotid Body Resection (CBR). In the CBR group, the carotid body on each side was visualised using a modular routine stereo microscope Leica M80 and surgically removed under ×25 magnification, using fine surgical forceps, with care taken to preserve fine branches of the carotid sinus nerve. Chemo‐ and baro‐reflex testing was repeated to verify that CBR was successful as reflected by an elimination of the chemoreflex‐evoked pressor response and preserving of the carotid baroreflex response. Finally, we cut the carotid sinus nerves bilaterally (CSNX) to complete a full sino‐aortic denervation. Baroreflex testing was then repeated to verify that the prior ADNX procedure had been performed successfully. In the Time Control group, only ADNX was performed followed by baro‐ and chemo‐receptor reflex testing at time intervals matching those of the study protocols.
Recovery surgical protocols
Rats were implanted with radio‐telemetry devices (PA‐C40; DSI, St. Paul, MN, USA) to record arterial pressure, and a chronic femoral vein catheter as described previously (Waki et al. 2006; McBryde et al. 2013; Pijacka et al. 2016). Briefly, under ketamine/medetomidine anaesthesia as described above, the arterial pressure catheter tip was inserted into the abdominal aorta below the level of the renal arteries, and secured in place. Non‐steroidal anti‐inflammatory pain relief was administered pre‐ and post‐operatively (0.004 ml/100 g of Metacam, Boehringer Ingelheim, Ingelheim, Germany). Animals were given at least 7 days of recovery before recording baseline. Femoral venous catheters were flushed with 0.9% saline/100 U heparin every second day to maintain their patency.
CBR was carried out under anaesthesia as described above. Like the non‐recovery protocol, the common carotid arteries were accessed through a midline neck incision. A 3.0 silk suture was used to retract the common carotid artery to improve visualisation and access to the bifurcation of the common carotid artery. Each common carotid artery was separated from the sternohyoid muscle and the carotid artery bifurcation was gently pulled away from the superior cervical ganglion; this allowed visualisation of the carotid body and the carotid sinus nerves. Using fine surgical forceps and under ×25 magnification the carotid body was surgically removed with care taken to preserve fine branches of the carotid sinus nerve.
Experimental protocol for conscious SH rat experimentation
After recovery from implantation surgery, blood pressure was recorded continuously (Spike2 version 8, CED, Cambridge, UK) for 1 week before (Baseline) and 2 weeks after both Sham (n = 9) and CBR (n = 9) surgeries. Data represent a weekly average for light (07.00–18.00 h) and dark (19.00–06.00 h) phases. On separate days, the cardiovascular responses to baroreflex and chemoreflex activation, moderate exercise (10 m min−1), hypoxia (10% O2, N2) and hypercapnia (7% CO2, 93% O2) were tested before and 2 weeks after Sham or CBR.
Baroreflex and chemoreflex tests
Bradycardic and tachycardic reflex responses were produced by ramp changes in arterial pressure as described above. A four‐parameter sigmoidal regression function was fitted to produce baroreflex function curves, using purpose‐written scripts in Spike2. The chemoreflex was tested as above.
Spontaneous baroreflex sensitivity and spectral analysis
Spontaneous baroreflex sensitivity (BRS) and spectral analysis parameter settings were used as described previously (Waki et al. 2006), and applied using the open access software CardioSeries v2.4 (http://www.danielpenteado.com). For BRS sequences, a series of at least four consecutive heart beats where there was a defined increase or decrease in systolic arterial pressure followed by a baroreflex response in pulse interval were used to fit linear regression curves; r 2 > 0.8. The spontaneous BRS is presented as the slope (ms mmHg−1) of the linear regression analysis between systolic blood pressure and pulse interval. For heart rate and systolic blood pressure spectral analysis, beat‐by‐beat series of pulse intervals and systolic blood pressure were converted to evenly spaced series using cubic spline interpolation (10 Hz) and divided into half‐overlapping sequential sets of 512 data points (Welch periodogram). Segments with transients that could affect the calculation of power spectral density were excluded. A Hanning window was used to attenuate side effects and the spectrum of each stationary segment was calculated using a fast Fourier transform (FFT) algorithm for discrete time series. The spectra of pulse intervals were integrated in low‐frequency (LF; 0.2–0.75 Hz) and high‐frequency (HF; 0.75–3 Hz) bands and the results are expressed as normalised units (nu) as described previously (Burr, 2007). The spectra of systolic arterial pressure were integrated only in low‐frequency (LF; 0.2–0.75 Hz) bands and the results are expressed in absolute (mmHg2) units. The LF/HF ratio was calculated to assess sympatho‐vagal balance.
Exercise test
The cardiovascular responses to forced moderate exercise were assessed in a purpose‐built motorised wheel. Rats were exercised for a total of 10 min, in a pattern consistent with voluntary exercise patterns: 40 s run/20 s break, at a speed of 10 m min−1 (Leasure & Jones, 2008) during their active phase, 19.00–21.00 h. Because it has been previously reported that chronic exercise training decreases resting blood pressure in the SH rat (Burger et al. 1998; Graham & Rush, 2004; Gu et al. 2015), we decided not to train our experimental animals. Rats were thus not previously exposed to the exercise wheel, so the exercise probably includes an element of stress.
Hypoxia and hypercapnia tests
Hypoxia and hypercapnia experiments were carried out in the afternoon between 12.00 and 16.00 h. Before experiments began, each rat was given at least 1 h to acclimatise to the chamber. The responses to hypoxia (10% oxygen, balance nitrogen, BOC) and hypercapnia (7% CO2, 93% O2, BOC) were tested in a normobaric chamber on the same animals on separate days. Baseline blood pressure, heart rate and respiratory rate were recorded during the delivery of humidified atmospheric air (21% O2/N2) followed by 15 min of exposure to either hypoxia or hypercapnia at a rate of 8 l min−1. Note, as shown herein and reported previously, hyperoxia fails to attenuate the response of the carotid bodies to hypercapnia in a variety of species including rat (Carroll & Bureau, 1988; Pepper et al. 1995; Rodman et al. 2001); hence, 7% CO2 was mixed with 93% oxygen.
Histology
On completion of the experimental protocol, animals were terminally anaesthetised with an overdose of sodium pentobarbital (100 mg kg−1) and the carotid bifurcations were removed and fixed (4% paraformaldehyde for 24 h, then stored in 30% sucrose/0.05% sodium azide). Using a cryostat, sections 10 μm thick were cut and mounted on Superfrost Plus slides, then stained with haematoxylin and eosin. Briefly, slides were stained with Ehrlich's haematoxylin for 3 min, washed, de‐stained in 1% acid alcohol for 20 s, washed and then counterstained with eosin for 10 s. Slides were then progressively dehydrated (70, 90 and 100% ethanol), immersed in xylene (3 × 5 min each) and covered with coverslips using a mounting medium. Images were obtained using a light microscope and ImageJ software.
Statistical analysis
Statistical analysis was conducted using SPSS (IBM SPSS version 23) and GraphPad v 6.0, and baroreflex curve and sigmoidal regression were performed in Spike2 software (version 8, CED) using purpose‐written scripts provided by CED. Responses during exercise, hypoxia and hypercapnia were analysed by two‐way ANOVA with repeated measures on two factors (time and intervention, before and after surgery). Post hoc tests used are reported in the corresponding figure legends. The factor analysed by the post hoc test is ‘Intervention’ and the data are compared at each time point before and after surgery within either the Sham or CBR group. Exercise, hypoxia and hypercapnia responses were also analysed by the area under the curve (AUC) method, which compared the area under the curve between before and after surgery. The statistical test performed is indicated in the figure legend. Data are presented as mean ± SEM, with a significance level of P < 0.05.
Results
Successful removal of the carotid bodies was confirmed using histochemistry on the carotid bifurcations removed from CBR rats; data were compared with sham rats (Fig. 1). Figure 1 shows an absence of glomus cells after CBR. An absence of the carotid body was found in all 15 rats that underwent CBR surgery. Carotid bodies were always found in sham rats (n = 14).
Figure 1. Histological confirmation of carotid body resection.
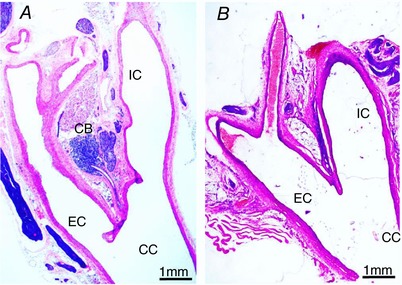
A, sham surgery with intact carotid body; B, carotid body resection (CBR) with absence of the peripheral chemoreceptor. Eosin and haematoxylin staining of representative images. CC, common carotid artery; IC, internal carotid artery; EC, external carotid artery; CB, carotid body. [Color figure can be viewed at http://wileyonlinelibrary.com]
Physiological validation of selective carotid body resection – anaesthetised rats
Eleven (5 Time Control; 6 CBR) anaesthetised male SH rats were used to confirm selective carotid body resection (CBR). The systolic blood pressure (SBP) response to chemoreflex activation was present, but significantly lower under anaesthesia than in the same rat when conscious (ΔSBP, Anaesthetized: 8 ± 2 mmHg vs. Conscious: 86 ± 8 mmHg; P < 0.001); however, a similar degree of bradycardia was observed (ΔHR, Anaesthetized: −99 ± 13 bpm vs. Conscious: −132 ± 12 bpm; P > 0.05).
Chemoreflex testing was performed during the baseline period after resection of the aortic depressor nerves (ADNX) and repeated after carotid body resection (CBR) or a sham procedure in the Time Control group. CBR abolished the chemoreflex response seen as a loss of the increase in SBP (P < 0.05; Fig. 2 B) and an absence of bradycardia (P < 0.05; Fig. 2 A). The Time Control group showed an increase in the SBP response over time (P < 0.05), which may reflect an increased sensitivity to repeated chemoreflex stimulation. However, the heart rate response was similar.
Figure 2. Assessment of chemoreflex and baroreflex sensitivity before and after progressive and selective chemo‐ and baro‐reflex denervation in anaesthetized SH rats.
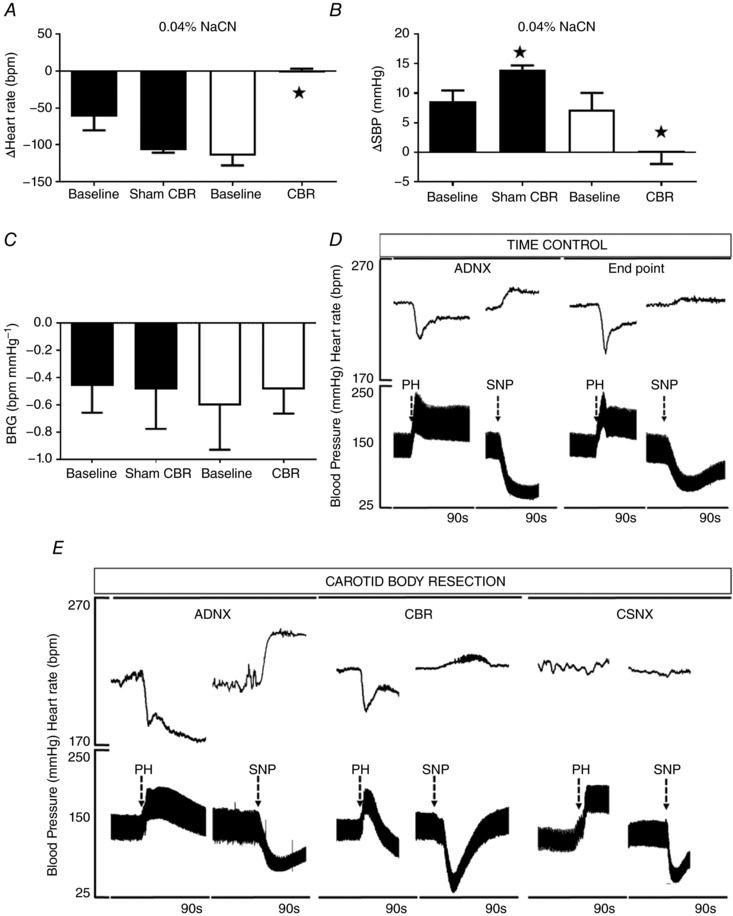
A and B, chemoreflex‐induced changes in (A) heart rate (HR) and (B) systolic blood pressure (SBP) by i.v. bolus infusion of sodium cyanide (0.04% NaCN) were abolished after carotid body resection (CBR) (P < 0.05). C, the cardiac baroreceptor reflex was preserved after CBR in the absence of aortic depressor nerves (ADNX); D and E, representative images illustrating the individual response to phenylephrine (PH) and sodium nitroprusside (SNP) in the time control and CBR group. CSNX resulted in abolishment of the baroreceptor reflex in the CBR group. Data were analysed by two‐way ANOVA with Sidak post hoc test. Sham n = 5, CBR = 6, * P < 0.05. Data are presented as mean ± SEM.
Baroreceptor reflex gain was preserved after combined ADNX and CBR (Fig. 2 C and D; n.s.). At the end of the experiment, rats in the CBR group underwent bilateral carotid sinus nerve denervation (CNSX), after which the heart rate baroreceptor reflex was completely abolished, confirming complete sino‐aortic denervation (Fig. 2 E).
Baseline changes in blood pressure after selective resection of the carotid bodies in conscious SH rats
Eighteen (9 Sham; 9 CBR) male SH rats were used to study the effect of the selective CBR on cardiovascular and respiratory parameters in conscious freely moving animals. Data are presented in Fig. 3 for both dark and light phases at baseline – week 0 (W0), 1 week (W1) and 2 weeks (W2) after CBR. A significant reduction in SBP was observed in the CBR group relative to baseline during both light and dark phases (P < 0.001; Fig. 3). Reductions in diastolic blood pressure (DBP) (P < 0.001), heart rate (P < 0.001) and respiratory rate (P < 0.001) also occurred in both light and dark phases (Fig. 3). Sham operated rats showed increases, when compared to baseline, in SBP (P < 0.05) and DBP (P < 0.05) recorded in the light phase (only) and an increase in respiratory rate (RR) in both phases (P < 0.05, Fig. 3).
Figure 3. Blood pressure and heart rate change after selective carotid body resection in conscious rats.
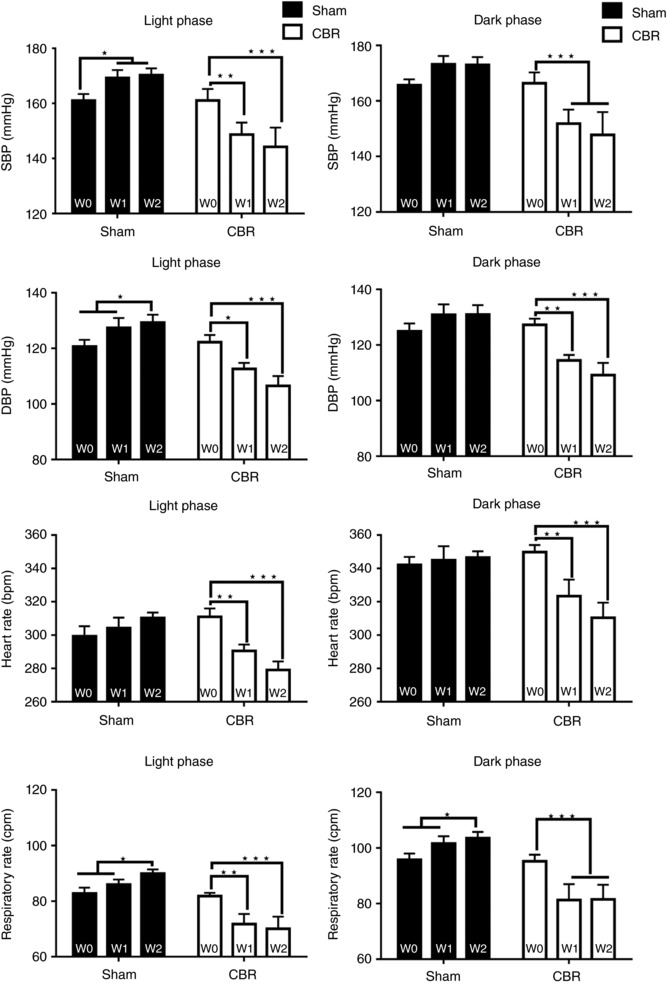
Cardiovascular responses to CBR in conscious SH rats. Temporal responses in systolic blood pressure (SBP), diastolic blood pressure (DBP), respiratory rate (RR) and heart rate (HR) are presented during light and dark phase. Data represent maximal response to the CBR or sham surgery recorded within the first (W1) and second (W2) week after treatment. W0, baseline. Data were analysed by two‐way ANOVA with Tukey post hoc test; n = 9; * P < 0.05, ** P < 0.01, *** P < 0.001. Data are presented as mean ± SEM.
Chemoreflex and baroreflex responses before and after selective carotid body resection in conscious rats
The arterial chemoreflex‐mediated pressor/bradycardia response, tested 2 weeks after surgery, was abolished after selective CBR (ΔSBP: before 79 ± 10 mmHg vs. after 9 ± 17 mmHg; ΔHR: before −130 ± 17 bpm vs. after −4 ± 4 bpm, P < 0.001; whereas the responses in the Sham operated animals remained (ΔSBP: before 92 ± 13 mmHg vs. after 84 ± 12 mmHg, ΔHR: before −134 ± 20 bpm vs. after −130 ± 17 bpm, n.s.; Fig. 4 A).
Figure 4. Chemo‐ and baro‐reflex function after selective carotid body resection (CBR) in the conscious SH rat.
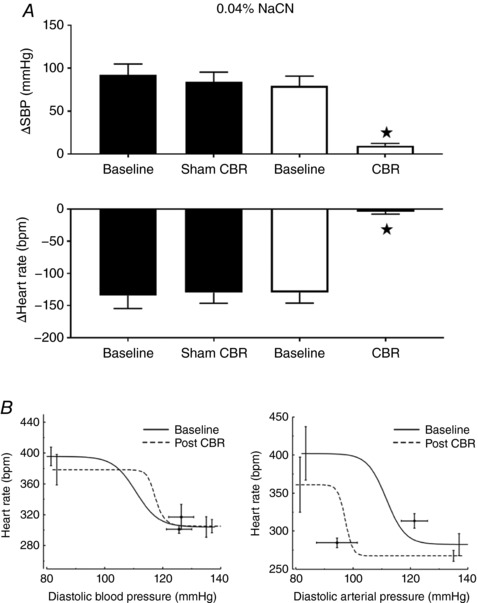
A, chemoreflex testing in the Sham and CBR groups. Surgical, selective, bilateral resection of the carotid bodies (CBR) abolished the chemoreflex response to 0.04% NaCN (SBP, systolic blood pressure; HR, heart rate; n = 7; P < 0.001). B, Sham (left) and CBR (right) group baroreflex test. The cardiac baroreflex function curve was shifted leftwards over lower pressure ranges after CBR in SH rats (right, P < 0.05). Data were analysed by two‐way ANOVA with Sidak post hoc test (A) and paired t test (n = 6, B); * P < 0.05. Data are presented as mean ± SEM.
Spontaneous baroreflex gain (sBRG) did not change after CBR (0.9 ± 0.09 ms mmHg−1 vs. 1.2 ± 0.29 ms mmHg−1; n.s.) or in sham rats (1.1 ± 0.17 ms mmHg−1 vs. 1.1 ± 0.1 ms mmHg−1; n.s.; Fig. 4 B). Sigmoidal baroreflex function curves showed no significant difference after CBR but a leftwards resetting of the operating points to the lower level of arterial pressure (Fig. 4 B, right).
Spectral analysis of pulse interval and SBP after CBR in conscious rats
Spectral analysis of the pulse interval (Fig. 5) showed that CBR was associated with a reduction in LF power (30.4 ± 1 nu vs. 23.2 ± 2 nu; P < 0.05) and an increase in HF power (69.6 ± 1 nu vs. 76.8 ± 2 nu; P < 0.05) resulting in a marked reduction in the LF/HF ratio (0.48 ± 0.03 vs. 0.33 ± 0.03; P < 0.05). Sham did not elicit significant changes in LF power (25.5 ± 2 nu vs. 30.2 ± 4 nu; n.s.), HF power (from 74.5 ± 2 nu to 70.3 ± 3 nu; n.s.) and LF/HF ratio (0.38 ± 0.04 nu vs. 0.5 ± 0.08 nu; n.s.). These results suggest that CBR leads to an improvement in cardiac sympatho‐vagal balance. Regarding SBP spectral analysis, we observed a significant reduction in the LF component of SBP in the CBR group (5.3 ± 0.9 mmHg2 vs. 1.9 ± 0.6 mmHg2; P < 0.05) but not in the Sham group (2.3 ± 0.5 mmHg2 vs. 3.2 ± 1.3 mmHg2; n.s.), suggesting a reduction in sympathetic vasomotor tone after CBR.
Figure 5. Effect of carotid body resection (CBR) on cardiac sympatho‐vagal balance in conscious SH rats.
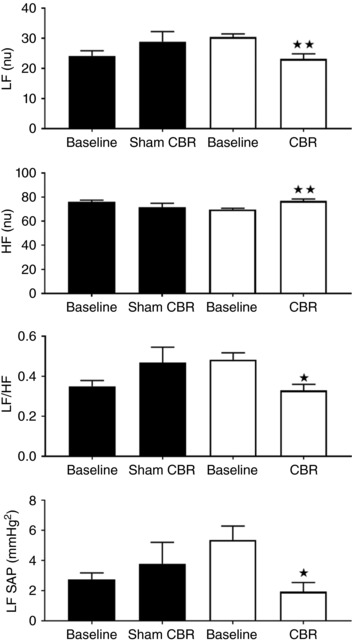
Sympatho‐vagal balance was unaffected in the Sham group (n.s.). CBR decreased LF (nu), increased HF (nu) and decreased the LF/HF ratio. CBR also reduced the LF spectra of SBP, suggesting sympatho‐inhibition. SBP, P < 0.05. Data were analysed by two‐way ANOVA with Sidak post hoc test (n = 6; * P < 0.05, ** P < 0.01). Data are presented as the mean ± SEM.
Exercise before and after selective resection of the carotid bodies
Sham and CBR rats did not show any differences in the ability to exercise; all groups showed an increase in blood pressure, heart rate and respiration (P < 0.001, Fig. 6 A and B). Our exercise challenge produced similar increases in blood pressure, heart rate and respiratory rates in sham and CBR rats (n.s.; Fig. 6 A and B). AUC analyses similarly showed that heart rate and respiration responses to exercise were not different between sham and CBR rats (n.s.; Fig. 6 A and B) but the pressor response in the Sham group increased after surgery (SBP, P < 0.05, Fig. 6 A).
Figure 6. Exercise challenges before and after selective carotid body resection (CBR) or sham surgery.
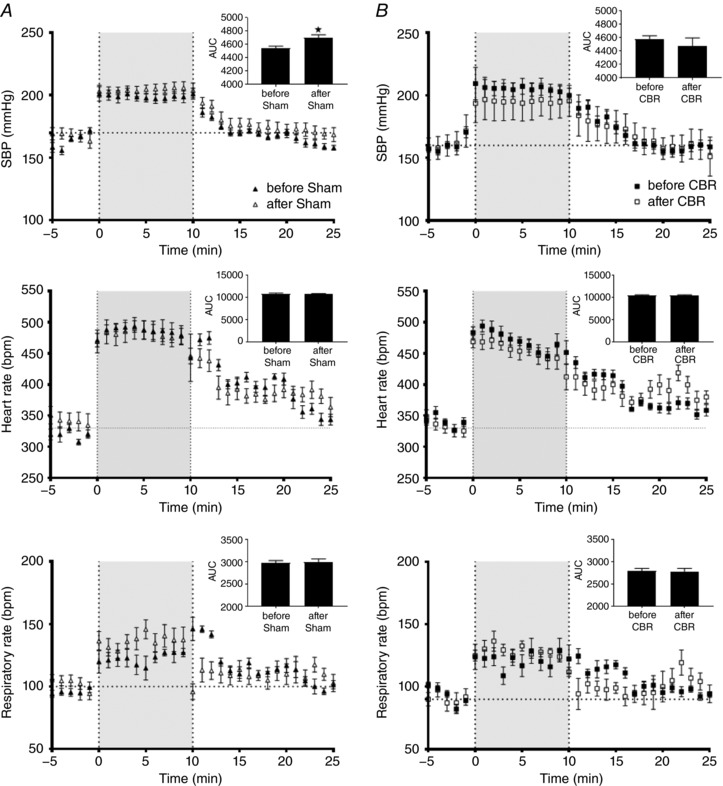
Exercise produced similar increases in systolic blood pressure (SBP), heart rate (HR) and respiratory rates (RR) in Sham (A) and CBR (B) rats over time (P < 0.001). Neither Sham nor CBR influenced the rat's ability to exercise (n.s.). Data were analysed by two‐way ANOVA with Sidak post hoc test comparing before vs. after surgery at each time point (n = 7). Additionally, the AUC (top right corner of each graph) showed that the pressor response in the Sham group increased after surgery (* P < 0.05). Data are presented as mean ± SEM.
Hypoxic challenge before and after selective resection of the carotid bodies
Two‐way ANOVA show that exposure to hypoxia (10% oxygen) produced a pressor response in sham animals, and in the CBR group before resection of carotid bodies, accompanied by an increase in heart rate and respiration (Fig. 7 A and B, P < 0.001). In contrast, CBR abolished the pressor response (SBP; P < 0.05) whereas responses in heart rate and respiratory rate were similar to before CBR animals (HR, RR; n.s.).
Figure 7. Hypoxia challenge before and after selective carotid body resection (CBR) or sham surgery.
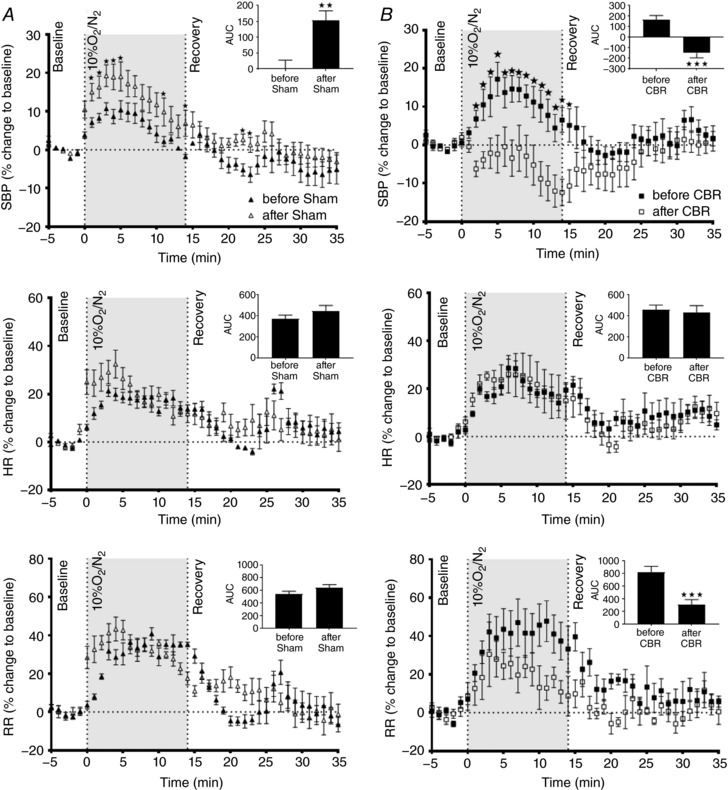
Exposure to 10% oxygen increased systolic blood pressure (SBP), heart rate (HR) and respiratory rate (RR) in (A) Sham group and (B) CBR group before surgery (P < 0.001). However, CBR abolished (P < 0.05) and Sham surgery further exacerbated (P < 0.05) the pressor response. CBR did not alter the response to hypoxia either in HR or in RR (n.s.). Data were analysed by two‐way ANOVA with Sidak post hoc test comparing before vs. after surgery at each time point (* P < 0.05; n = 7). Moreover, the AUC (top right corner of each graph) confirmed the SBP responses and identified that the RR decreased after CBR (** P < 0.01 and *** P < 0.001). Data are presented as mean ± SEM.
Following the sham surgery, animals showed an increase in the pressor response to hypoxia (SBP; P < 0.05) but responses in heart rate and respiratory rate were similar to before Sham (n.s.).
Statistical analyses of the AUCs confirmed these results. Interestingly, AUC analyses also showed that the respiratory rate decreased after CBR (P < 0.001).
Hypercapnia challenge before and after selective resection of the carotid bodies
Exposure to 7% CO2/93% O2 resulted in an increase in SBP, heart rate and respiratory rate in all groups (P < 0.001, Fig. 8 A and B). The SBP response to hypercapnia was augmented after CBR (P < 0.01, Fig. 8 B). The responses in the Sham group did not differ before and after surgery (n.s., Fig. 8 A). Analyses performed on AUC confirmed these responses. Additionally, it identified that respiratory rate increased after CBR (P < 0.01).
Figure 8. Hypercapnia challenge before and after selective carotid body resection (CBR) or sham surgery.
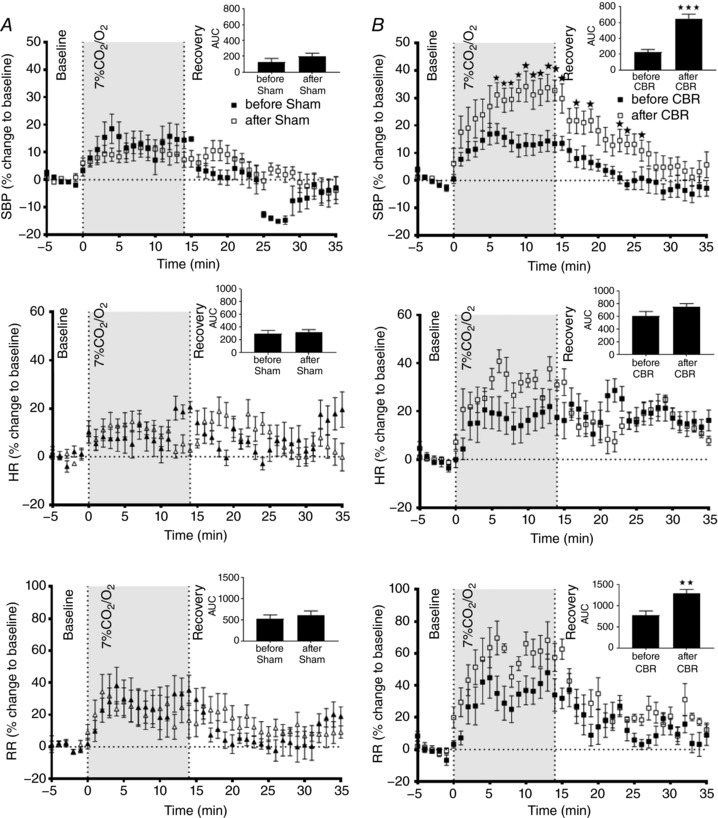
Exposure to 7% CO2/93% O2 produced a pressor response in all animals, accompanied by an increase in heart rate and respiration (P < 0.001). After CBR the pressor response was augmented relative to the before CBR group (P < 0.01). CBR did not alter the response in heart rate (HR) to hypercapnia and the respiratory rates (RR; n.s.). Data were analysed by two‐way ANOVA with Sidak post hoc test comparing before vs. after surgery at each time point (* P < 0.05; n = 7). Moreover, the analysis on AUC (top right corner of each graph) confirmed the CBR effect on SBP. Additionally, it showed that RR increased after CBR (** P < 0.01 and ***P < 0.001). Data are presented as mean ± SEM.
Discussion
We demonstrate for the first time that the carotid bodies can be surgically resected while preserving carotid sinus baroreflex function in the SH rat for at least 2 weeks. We have carefully validated our surgical approach to confirm both that: (i) the carotid bodies were removed, (ii) carotid sinus baroreceptor function was preserved and not dissimilar to sham animals, and (iii) carotid chemoreceptor reflex function was abolished. Chronic blood pressure recordings indicated that selective CBR produced a sustained and significant reduction in arterial pressure in the SH rat across both light and dark phases; the magnitude of this reduction was consistent with our previously reported findings where the carotid sinus nerves were denervated bilaterally (Franchini & Krieger, 1992; Abdala et al. 2012; McBryde et al. 2013), and involved reductions in sympathetic drive to both the heart and the vasculature, as measured indirectly with spectral analysis. In these hypertensive rats, we also revealed an essential role of the carotid chemoreflex in mediating the pressor response to hypoxia. Notably, the pronounced pressor response to hypoxia was absent after CBR. In contrast, we found that the cardiovascular response to exercise was unchanged after CBR, suggesting either that the carotid bodies do not play a critical role in mediating this response, or that compensation by alternative pathways occurred. Finally, the pressor response to hypercapnia was augmented after CBR.
Our ability to selectively remove the carotid bodies is an important advance, as previous techniques by ourselves and others (Abdala et al. 2012; Del Rio et al. 2013; McBryde et al. 2013; Marcus et al. 2014; Iturriaga et al., 2015a; Pijacka et al. 2016) have relied on stripping the carotid sinus of all nerves, thus removing baro‐receptive as well as chemo‐receptive afferents. Impressively, despite the bilateral denervation of the carotid sinus baroreceptors found in our previously published studies, a functional baroreflex was observed to be maintained in these animals, presumably via compensation from the aortic depressor baroreceptor pathway (Abdala et al. 2012; McBryde et al. 2013). Our current results extend this previous work, showing for the first time that following specific CBR when a fully functional baroreflex is maintained (i.e. carotid sinus and aortic), with a leftward shift resetting around the lower level of arterial pressure, a substantial anti‐hypertensive response persists.
Given the evidence of raised carotid body activity during exercise (Jacobi et al. 1989; Ward, 1994) and improved exercise tolerance after CBR in humans (Niewinski et al. 2017), we were surprised to find no difference in the cardiovascular response after CBR. Although the cardiovascular responses were not reported, Lugliani et al. (1971) showed that the respiratory responses to moderate steady‐state exercise were not affected by CBR, consistent with our finding that the cardiovascular responses to exercise are not reliant on input from the carotid bodies, at least in the SH rat. However, we acknowledge that the exercise protocol we used was not without environmental stress as the animals were forced to run in an enclosed motorised running wheel. Furthermore, we chose not to condition the animals to the running wheel, as carotid body sensitivity is reduced with exercise training (Burger et al. 1998; Graham & Rush, 2004; Gu et al. 2015). Thus, the absence of an effect of CBR on the blood pressure and heart rate responses during exercise in our study may include a stress component. Therefore, the effect of CBR on blood pressure control during exercise in SH rats remains equivocal.
Our observation that CBR blunts the ventilatory response and reverses the pressor response to hypoxia is consistent with recent studies in human patients with heart failure, who underwent bilateral CBR (Niewinski et al. 2014). In keeping with our current results, Niewinski et al. (2014) showed that CBR reduced the respiratory and arterial pressure responses to hypoxia, whilst the heart rate response was unchanged. Similarly, early human studies in which bilateral CBR was performed to treat bronchial asthma found that the respiratory response to hypoxia was absent (Lugliani et al. 1971). Our data in SH rats and in humans may have important implications when evaluating the carotid body as a potential therapeutic target in cardiovascular disease, as subjects lacking carotid bodies may be less able to cope with situations where oxygen availability is decreased. This is borne out by the recent observation of worsening blood oxygen saturations at night in heart failure patients after bilateral CBR (Niewinski et al. 2017). This supports our contention that carotid body therapy should modulate, not abolish, its function (Pijacka et al. 2016).
We performed bilateral CBR because unilateral carotid sinus denervation was ineffective in lowering blood pressure in SH rats (McBryde et al. 2013). In contrast, unilateral carotid body denervation in drug‐resistant hypertensive patients was effective in ∼60% of patients tested, suggesting a possible species difference. In our study (Narkiewicz et al. 2016) and that of others (Limberg et al. 2015), unilateral carotid body ablation lowered arterial pressure in some patients, which was well maintained at 3 and 6 months of follow‐up with some showing a relapse by 12 months; the relapse may reflect compensation from the contralateral carotid body. Nevertheless, preservation of the contralateral carotid body may be necessary to preserve protection against hypoxia in these patients, especially during sleep. This view is supported by a recent case report examining various sympatho‐excitatory reflex tests in a patient with (prior) unilateral carotid body resection for paraganglioma (Larson et al. 2017). They reported that hypoxic ventilatory responses were normal, but that the sympatho‐excitatory responses to static exercise appeared to be blunted.
Exposing rats without carotid bodies to hyperoxic hypercapnia produced an exaggerated pressor response compared to sham controls. We propose that this is due to a greater plasma level of CO2 that results from a reduced ventilatory response; this is borne out by the reduced breathing frequency response to hypercapnia after CBR. We presume the plasma contains an elevated level of CO2 that provides a greater stimulus to the central chemoreceptors. We recognise that this will need to be confirmed using blood sampling, which was not tenable in the present study. We acknowledge that the use of hyperoxia might have: (i) suppressed basal discharge of the carotid bodies in the sham control group and (ii) caused a confounding vasoconstrictive effect. However, this would be expected to be the same in the sham and CBR groups, making their comparison relative. Also, hyperoxia does not attenuate the response of the carotid bodies to hypercapnia in the rat (Carroll & Bureau, 1988; Pepper et al. 1995; Rodman et al. 2001) so this is not problematic. All told, the exaggerated rise in blood pressure to hypercapnia after CBR is potentially worrisome and could pose problems clinically in terms of inducing stroke.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
JFRP was responsible for acquisition of funding, administrative support, study conception, design of the experiments and drafting the manuscript. WP designed the experiments, and collected, analysed and interpreted the data. FDM analysed and interpreted the BRG data. Both WP and FDM wrote the manuscript. PLK performed and interpreted the spectral analsyis. GSL and PLK performed immunohistochemistry. HCS and RRC contributed to the editing of the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Funding
The study was funded by a programme grant awarded to JFRP from the British Heart Foundation (RG/12/6/29670).
Translational Perspective
The present study raises potentially clinically relevant problems with bilateral carotid body resection. Although there are positive effects on blood pressure control in conditions of hypertension, the SH rat was not able to control blood pressure after CBR when exposed to hypoxia and exhibited excessive rises in blood pressure to hypercapnia. These could trigger end organ damage and may be particularly pertinent to human patients with sleep apnoea. Through extension, it might be expected that in other situations where the carotid bodies would normally be engaged, the homeostatic control of blood pressure and ventilation may become jeopardised. Given that the carotid body has multiple other functions, such as blood glucose control (Limberg et al. 2014; Sacramento et al. 2017), multiple levels of organ and systems failure could occur under different states of health and disease without carotid bodies. We surmise that blunt resection is not optimal and efforts are now needed to find pharmacological approaches that can normalise carotid body function by abolishing hyperreflexia and tonicity without destroying physiological function; the purinergic P2X3 receptors are one such example that we have proposed (Pijacka et al. 2016) but others have also been suggested, including anti‐oxidant therapy (Iturriaga et al. 2015a,b) and caffeine, which is known to block adenosine receptors and decrease carotid body sensitisation following chronic intermittent hypoxia (Sacramento et al. 2015). The relevance of the latter is that habitual coffee drinking was found to lower blood pressure, especially in women (Geleijnse, 2008).
Acknowledgements
We acknowledge Dr Jean‐Charles Isner (University of Bristol) for designing and building the motorised wheel for the forced exercise test. The European Union, Seventh Framework Programme, Marie Curie Actions (CARPEDIEM—No 612280) also supported this study.
Biography
Wioletta Pijacka is working as a Senior Research Associate at the University of Bristol. Her work has focused on identifying new therapeutic targets for blood pressure control. More specifically, she is interested in the role of the carotid body in the pathological increase of sympathetic activity leading to high blood pressure by using animal models of neurogenic and renovascular hypertension. She recently characterised the role of a purinergic receptor in this pathology. Here she presents a new approach for the selective resection of the carotid body that spares the carotid sinus baroreceptors and reduces blood pressure in hypertensive rats.

Edited by: Harold Schultz & Frank Powell
This is an Editor's Choice article from the 1 August 2018 issue.
Linked articles This article is highlighted by a Perspective by Conde. To read this Perspective, visit https://doi.org/10.1113/JP275796.
References
- Abdala AP, McBryde FD, Marina N, Hendy EB, Engelman ZJ, Fudim M, Sobotka PA, Gourine AV & Paton JF (2012). Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J Physiol 590, 4269–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade DC, Lucero C, Toledo C, Madrid C, Marcus NJ, Schultz HD & Del Rio R (2015). Relevance of the carotid body chemoreflex in the progression of heart failure. BioMed Res Int 2015, 467597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger HR, Chandler MP, Rodenbaugh DW & DiCarlo SE (1998). Dynamic exercise shifts the operating point and reduces the gain of the arterial baroreflex in rats. Am J Physiol Regul Integr Comp Physiol 275, R2043–2048. [DOI] [PubMed] [Google Scholar]
- Burr RL (2007). Interpretation of normalized spectral heart rate variability indices in sleep research: a critical review. Sleep 30, 913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JL & Bureau MA (1988). Peripheral chemoreceptor CO2 response during hyperoxia in the 14‐day‐old awake lamb. Respir Physiol 73, 339–349. [DOI] [PubMed] [Google Scholar]
- Chu AL, Jay O & White MD (2007). The effects of hyperthermia and hypoxia on ventilation during low‐intensity steady‐state exercise. Am J Physiol Regul Integr Comp Physiol 292, R195–203. [DOI] [PubMed] [Google Scholar]
- Del Rio R, Marcus NJ & Schultz HD (2013). Carotid chemoreceptor ablation improves survival in heart failure: rescuing autonomic control of cardiorespiratory function. J Am Coll Cardiol 62, 2422–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ & Farquhar WB (2010). Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 299, H1318–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiamma MN, O'Connor ET, Roy A, Zuna I & Wilson RJ (2013). The essential role of peripheral respiratory chemoreceptor inputs in maintaining breathing revealed when CO2 stimulation of central chemoreceptors is diminished. J Physiol 591, 1507–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini KG & Krieger EM (1992). Carotid chemoreceptors influence arterial pressure in intact and aortic‐denervated rats. Am J Physiol Regul Integr Comp Physiol 262, R677–683. [DOI] [PubMed] [Google Scholar]
- Geleijnse JM (2008). Habitual coffee consumption and blood pressure: an epidemiological perspective. Vasc Health Risk Manag 4, 963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DA & Rush JW (2004). Exercise training improves aortic endothelium‐dependent vasorelaxation and determinants of nitric oxide bioavailability in spontaneously hypertensive rats. J Appl Physiol (1985) 96, 2088–2096. [DOI] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Zhao L, Ma YP & Liu JD (2015). Contribution of mitochondrial function to exercise‐induced attenuation of renal dysfunction in spontaneously hypertensive rats. Mol Cell Biochem 406, 217–225. [DOI] [PubMed] [Google Scholar]
- Itskovitz J, LaGamma EF, Bristow J & Rudolph AM (1991). Cardiovascular responses to hypoxemia in sinoaortic‐denervated fetal sheep. Pediatr Res 30, 381–385. [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Andrade DC & Del Rio R (2015a). Crucial role of the carotid body chemoreceptors on the development of high arterial blood pressure during chronic intermittent hypoxia. Adv Exp Med Biol 860, 255–260. [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Moya EA & Del Rio R (2015b). Inflammation and oxidative stress during intermittent hypoxia: the impact on chemoreception. Exp Physiol 100, 149–155. [DOI] [PubMed] [Google Scholar]
- Jacobi MS, Patil CP & Saunders KB (1989). The transient ventilatory response to carbon dioxide at rest and in exercise in man. Respir Physiol 77, 225–237. [DOI] [PubMed] [Google Scholar]
- Kanbar R, Stornetta RL, Cash DR, Lewis SJ & Guyenet PG (2010). Photostimulation of Phox2b medullary neurons activates cardiorespiratory function in conscious rats. Am J Respir Crit Care Med 182, 1184–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulandavelu S, Balkan W & Hare JM (2015). Regulation of oxygen delivery to the body via hypoxic vasodilation. Proc Natl Acad Sci USA 112, 6254–6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson KF, Limberg JK, Baker SE, Joyner MJ & Curry TB (2017). Intact blood pressure, but not sympathetic, responsiveness to sympathoexcitatory stimuli in a patient with unilateral carotid body resection. Physiol Rep 5, ii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure JL & Jones M (2008). Forced and voluntary exercise differentially affect brain and behavior. Neuroscience 156, 456–465. [DOI] [PubMed] [Google Scholar]
- Lim PO, MacFadyen RJ, Clarkson PB & MacDonald TM (1996). Impaired exercise tolerance in hypertensive patients. Ann Intern Med 124, 41–55. [DOI] [PubMed] [Google Scholar]
- Limberg JK, Taylor JL, Dube S, Basu R, Basu A, Joyner MJ & Wehrwein EA (2014). Role of the carotid body chemoreceptors in baroreflex control of blood pressure during hypoglycaemia in humans. Exp Physiol 99, 640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberg JK, Taylor JL, Mozer MT, Dube S, Basu A, Basu R, Rizza RA, Curry TB, Joyner MJ & Wehrwein EA (2015). Effect of bilateral carotid body resection on cardiac baroreflex control of blood pressure during hypoglycemia. Hypertension 65, 1365–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincevicius GS, Shimoura CG, Nishi EE, Perry JC, Casarini DE, Gomes GN, Bergamaschi CT & Campos RR (2015). Aldosterone contributes to sympathoexcitation in renovascular hypertension. Am J Hypertens 28, 1083–1090. [DOI] [PubMed] [Google Scholar]
- Linton RA & Band DM (1985). The effect of potassium on carotid chemoreceptor activity and ventilation in the cat. Respir Physiol 59, 65–70. [DOI] [PubMed] [Google Scholar]
- Lugliani R, Whipp BJ, Seard C & Wasserman K (1971). Effect of bilateral carotid‐body resection on ventilatory control at rest and during exercise in man. N Engl J Med 285, 1105–1111. [DOI] [PubMed] [Google Scholar]
- Marcus NJ, Del Rio R, Schultz EP, Xia XH & Schultz HD (2014). Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J Physiol 592, 391–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JM & Metcalfe JD (1988). Analysis of the cardiovascular changes induced in the rat by graded levels of systemic hypoxia. J Physiol 407, 385–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBryde FD, Abdala AP, Hendy EB, Pijacka W, Marvar P, Moraes DJ, Sobotka PA & Paton JF (2013). The carotid body as a putative therapeutic target for the treatment of neurogenic hypertension. Nat Commun 4, 2395. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP & Iwamoto GA (1983). The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45, 229–242. [DOI] [PubMed] [Google Scholar]
- Moraes DJ, Machado BH & Paton JF (2015). Carotid body overactivity induces respiratory neurone channelopathy contributing to neurogenic hypertension. J Physiol 593, 3055–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkiewicz K, Ratcliffe LE, Hart EC, Briant LJ, Chrostowska M, Wolf J, Szyndler A, Hering D, Abdala AP, Manghat N, Burchell AE, Durant C, Lobo MD, Sobotka PA, Patel NK, Leiter JC, Engelman ZJ, Nightingale AK & Paton JF (2016). Unilateral carotid body resection in resistant hypertension: a safety and feasibility trial. JACC Basic Transl Sci 1, 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewinski P, Janczak D, Rucinski A, Jazwiec P, Sobotka PA, Engelman ZJ, Fudim M, Tubek S, Jankowska EA, Banasiak W, Hart EC, Paton JF & Ponikowski P (2013). Carotid body removal for treatment of chronic systolic heart failure. Int J Cardiol 168, 2506–2509. [DOI] [PubMed] [Google Scholar]
- Niewinski P, Janczak D, Rucinski A, Tubek S, Engelman ZJ, Jazwiec P, Banasiak W, Sobotka PA, Hart EC, Paton JF & Ponikowski P (2014). Dissociation between blood pressure and heart rate response to hypoxia after bilateral carotid body removal in men with systolic heart failure. Exp Physiol 99, 552–561. [DOI] [PubMed] [Google Scholar]
- Niewinski P, Janczak D, Rucinski A, Tubek S, Engelman ZJ, Piesiak P, Jazwiec P, Banasiak W, Fudim M, Sobotka PA, Javaheri S, Hart EC, Paton JF & Ponikowski P (2017). Carotid body resection for sympathetic modulation in systolic heart failure: results from first‐in‐man study. Eur J Heart Fail 19, 391–400. [DOI] [PubMed] [Google Scholar]
- Oikawa S, Hirakawa H, Kusakabe T, Nakashima Y & Hayashida Y (2005). Autonomic cardiovascular responses to hypercapnia in conscious rats: the roles of the chemo‐ and baroreceptors. Auton Neurosci 117, 105–114. [DOI] [PubMed] [Google Scholar]
- Paterson DJ (1996). Role of potassium in the regulation of systemic physiological function during exercise. Acta Physiol Scand 156, 287–294. [DOI] [PubMed] [Google Scholar]
- Paton JF, Ratcliffe L, Hering D, Wolf J, Sobotka PA & Narkiewicz K (2013). Revelations about carotid body function through its pathological role in resistant hypertension. Curr Hypertens Rep 15, 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper DR, Landauer RC & Kumar P (1995). Postnatal development of CO2‐O2 interaction in the rat carotid body in vitro . J Physiol 485, 531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper DR, Landauer RC & Kumar P (1996). Extracellular potassium and chemosensitivity in the rat carotid body, in vitro . J Physiol 493, 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering AE, Simms AE & Paton JF (2008). Dominant role of aortic baroreceptors in the cardiac baroreflex of the rat in situ. Auton Neurosci 142, 32–39. [DOI] [PubMed] [Google Scholar]
- Pijacka W, Moraes DJ, Ratcliffe LE, Nightingale AK, Hart EC, da Silva MP, Machado BH, McBryde FD, Abdala AP, Ford AP & Paton JF (2016). Purinergic receptors in the carotid body as a new drug target for controlling hypertension. Nat Med 22, 1151–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe LE, Pijacka W, McBryde FD, Abdala AP, Moraes DJ, Sobotka PA, Hart EC, Narkiewicz K, Nightingale AK & Paton JF (2014). CrossTalk opposing view: Which technique for controlling resistant hypertension? Carotid chemoreceptor denervation/modulation. J Physiol 592, 3941–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodman JR, Curran AK, Henderson KS, Dempsey JA & Smith CA (2001). Carotid body denervation in dogs: eupnea and the ventilatory response to hyperoxic hypercapnia. J Appl Physiol (1985) 91, 328–335. [DOI] [PubMed] [Google Scholar]
- Sabino JP, Oliveira M, Giusti H, Glass ML, Salgado HC & Fazan R Jr (2013). Hemodynamic and ventilatory response to different levels of hypoxia and hypercapnia in carotid body‐denervated rats. Clinics (Sao Paulo) 68, 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacramento JF, Gonzalez C, Gonzalez‐Martin MC, Conde SV (2015). Adenosine receptor blockade by caffeine inhibits carotid sinus nerve chemosensory activity in chronic intermittent hypoxic animals. Adv Exp Med Biol 860, 133–137. [DOI] [PubMed] [Google Scholar]
- Sacramento JF, Ribeiro MJ, Rodrigues T, Olea E, Melo BF, Guarino MP, Fonseca‐Pinto R, Ferreira CR, Coelho J, Obeso A, Seica R, Matafome P & Conde SV (2017). Functional abolition of carotid body activity restores insulin action and glucose homeostasis in rats: key roles for visceral adipose tissue and the liver. Diabetologia 60, 158–168. [DOI] [PubMed] [Google Scholar]
- Schultz HD & Marcus NJ (2012). Heart failure and carotid body chemoreception. Adv Exp Med Biol 758, 387–395. [DOI] [PubMed] [Google Scholar]
- Sinski M, Lewandowski J, Przybylski J, Bidiuk J, Abramczyk P, Ciarka A & Gaciong Z (2012). Tonic activity of carotid body chemoreceptors contributes to the increased sympathetic drive in essential hypertension. Hypertens Res 35, 487–491. [DOI] [PubMed] [Google Scholar]
- Smith SA, Williams MA, Leal AK, Mitchell JH & Garry MG (2006). Exercise pressor reflex function is altered in spontaneously hypertensive rats. J Physiol 577, 1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein P, White SE, Homan J, Hanson MA & Bocking AD (1999). Altered fetal cardiovascular responses to prolonged hypoxia after sinoaortic denervation. Am J Physiol Regul Integr Comp Physiol 276, R340–346. [DOI] [PubMed] [Google Scholar]
- Takakura AC & Moreira TS (2011). Contribution of excitatory amino acid receptors of the retrotrapezoid nucleus to the sympathetic chemoreflex in rats. Exp Physiol 96, 989–999. [DOI] [PubMed] [Google Scholar]
- Vidruk EH, Olson EB Jr, Ling L & Mitchell GS (2001). Responses of single‐unit carotid body chemoreceptors in adult rats. J Physiol 531, 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waki H, Katahira K, Polson JW, Kasparov S, Murphy D & Paton JF (2006). Automation of analysis of cardiovascular autonomic function from chronic measurements of arterial pressure in conscious rats. Exp Physiol 91, 201–213. [DOI] [PubMed] [Google Scholar]
- Ward SA (1994). Peripheral and central chemoreceptor control of ventilation during exercise in humans. Can J Appl Physiol 19, 305–333. [DOI] [PubMed] [Google Scholar]


