Abstract
The health effects of inhaled electronic cigarette (e-cigarette) flavoring compounds are largely unknown. Earlier reports of their chemical reactivity have been conflicting, with some claiming, for example, that the degradation of flavoring chemicals in e-cigarettes to aldehydes is statistically insignificant. It is thus important to understand how these molecules react to afford enhanced aerosol products. The purpose of the current study was to investigate the origin of formaldehyde, acrolein, and acetaldehyde in e-cigarettes that contain the popular additive, triacetin (TA). By using 13C labeling and a combination of 1H NMR and 13C NMR, we were able to identify that ester hydrolysis of TA occurs to form acetic acid (HOAc) during aerosolization. The released HOAc acts as a catalyst in the degradation of propylene glycol (PG) and glycerol (GLY), increasing the formation of formaldehyde hemiacetals, acrolein, and acetaldehyde. A solution of 10% TA in 1:1 PG/GLY e-liquid was aerosolized using two different e-cigarettes at two wattages. Each device exhibited a significant increase in aldehyde levels, of up to 185% compared to the aerosol from a 1:1 PG/GLY e-liquid. In addition, the GLY formaldehyde hemiacetal was more predominant within the presence of HOAc, indicating that GLY may be relatively more prone to degradation from protonation.
Introduction
There are approximately 3 million adolescents using electronic cigarettes (e-cigarettes) in the United States.1 Moreover, e-cigarette usage has been reported to be a major risk factor among youth for traditional cigarette usage.2 Flavors are well-known to be a major contributing factor to the appeal of e-cigarettes,3,4 particularly among young people.5,6 Among US current e-cigarette users, 82% of young people and 70% of older adults use flavored e-cigarette liquid (e-liquid).7 The Food and Drug Administration (FDA) has yet to pass federal regulation on e-liquid flavoring chemicals.8 Research is needed to better understand the identity, levels, reactivity, and inhalation toxicology of specific flavor compounds.
E-liquid is typically composed of a mixture of carrier solvents, nicotine, and flavoring compounds. Many flavorings are listed as generally recognized as safe (GRAS) by the FDA as food additives for ingestion. However, their inhalation toxicity is largely unknown. Despite this, some vaping industry websites claim that e-liquids are safe for inhalation because of their GRAS rating.9,10
In addition to the lack of inhalation toxicity data, the chemical reactivity of the flavoring compounds used in e-cigarettes has also not been extensively investigated. Previous studies have shown that the aerosolization of flavored e-liquid increases toxic aldehyde production,11 oxidative stress,12,13 and inflammatory responses.14,15 Khlystov and Samburova compared the aldehyde production of flavored e-liquid to that of the aerosol derived from carrier e-liquid [(propylene glycol (PG) and glycerol (GLY)]. They identified a direct relationship between enhanced aldehyde levels and flavor compound concentration.11 Others have found that different commercial e-liquid flavoring formulations produced varying aerosol product profiles.16,17 However, to date, apart from the determination of sugar-derived furans in e-cigarette aerosols,18 there have been no reports focusing on how aerosol products derive from flavoring additives. For example, it is not known if enhanced levels of aldehydes derive directly from the flavoring molecules themselves or if flavorings promote the degradation of other e-liquid components such as the solvents PG/GLY. Herein, we used 13C labeling to unambiguously determine the origin of enhanced aldehyde levels from a relatively common e-liquid additive, triacetin (TA), the triester of GLY (i.e., glycerin triacetate, 1,2,3-triacetoxypropane). In addition to e-cigarette products, TA is also found in traditional cigarettes and cigars.19
TA is mainly used to enhance the overall flavor of the e-cigarette aerosol. It has become popular in the “do-it-yourself” community because of its ability to lessen the “bite”.20 Manufactures are not required to report TA’s presence or levels in e-liquids, so its abundance in e-liquids is largely unknown. However, we found three manufacturer websites that do report TA (Table 1, see also Table S3 in the Supporting Information). Importantly, some companies have also begun to use it as a replacement solvent for PG.21
Table 1. TA Reported in Various E-Liquid Flavors.
| vendor | flavors which are reported to contain TA | total flavors available | frequency (%) |
|---|---|---|---|
| The Flavor Apprentice | 19 | 443 | 4.3 |
| Flavor West | 24 | 338 | 7.1 |
| Simply Flavors | 51 | 150 | 34.0 |
Results and Discussion
Determination of the Aerosol Product Profiles
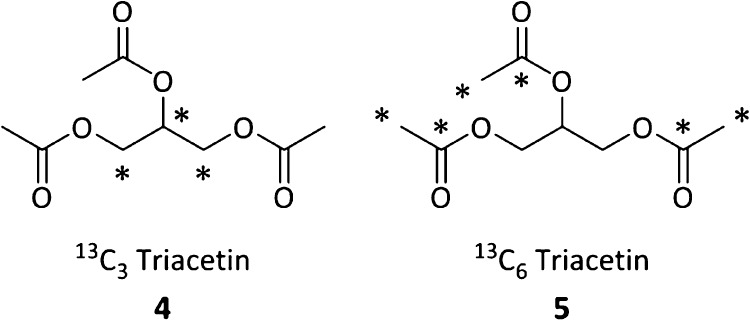
The two e-cigarette devices EC1 and EC2 were chosen to represent different coil options, namely, a sub-Ohm vertical coil (EC1) and a horizontal coil (EC2).22 Each was tested at two battery power settings that were chosen from self-described user preferences (Supporting Information) that were also within the manufacturers’ recommended ranges. In order to determine the origin of aldehyde aerosol products from the TA-containing e-liquid, we synthesized 13C-labeled TA from the reaction of GLY and acetic anhydride. Compound 4 (Figure 1) was derived from 13C-labeled GLY and compound 5 (Figure 1) from 13C-labeled acetic anhydride. The use of 13C-labeled TA molecules allowed us to determine whether TA forms aldehydes directly via its thermal decomposition (Scheme 1), or if TA plays a different role.
Figure 1.
Isotopically labeled TA. TA derived from isotopically labeled GLY (4) and from isotopically labeled acetic anhydride (5).
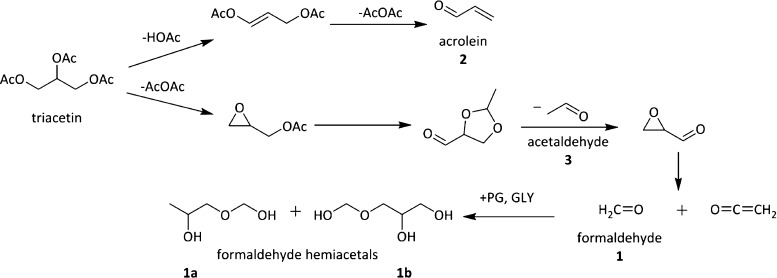
Scheme 1. Two Pathways of TA-Proposed Thermal Degradation.
TA forms acrolein, acetaldehyde, and formaldehyde. In e-cigarettes, formaldehyde further reacts with PG/GLY to form formaldehyde hemiacetals.23,26
TA has been reported23 to degrade under thermal conditions to form acetic acid (HOAc), formaldehyde, acrolein (2), and acetaldehyde (3), as shown in Scheme 1. The IARC (International Agency for Research on Cancer) reports formaldehyde as a known carcinogen and acetaldehyde as a possible carcinogen.24 Acrolein is a notorious air pollutant. It has been shown to cause a decrease in respiratory rates and to cause intense eye and respiratory irritation in humans. It has been shown to lead to inflammation, obstruction of the trachea and bronchi, and hemorrhaging in animals.25 Previously, 1–3 have been identified in the aerosol of e-cigarettes from the dehydration and oxidation of the e-liquid solvents.26
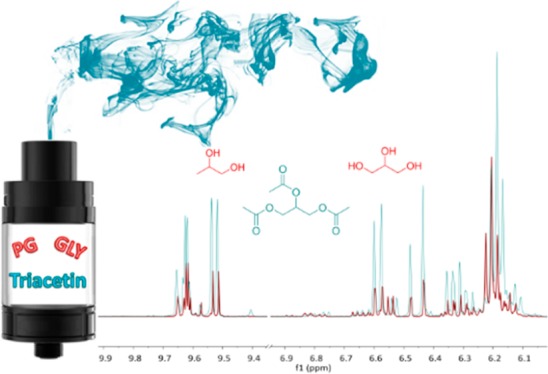
On the basis of the literature precedent,11,23 we anticipated an enhanced level of aldehyde byproducts in the aerosol derived from the flavored (i.e., TA-containing) e-liquid as compared to the aerosol from the e-liquid composed of PG/GLY alone. Indeed, an overlay of the 1H NMR spectra (Figure 2) of the aerosol derived from each type of e-liquid clearly shows that the aerosol derived from the TA/PG/GLY e-liquid contained higher levels of aldehydes 1a–b (as the formaldehyde hemiacetals),27 as well as 2 (acrolein) and 3 (acetaldehyde).
Figure 2.
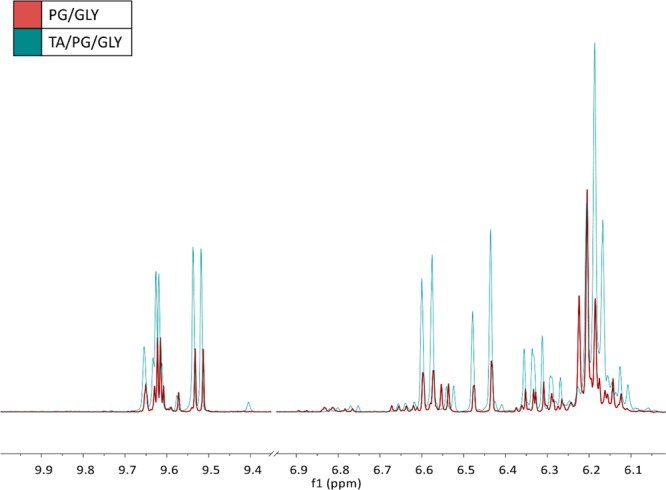
Overlay of the 1H NMR spectra of aerosolized (red) PG/GLY e-liquid and (blue) 10% TA/PG/GLY e-liquid. The peaks of interest that increase in height are identified by the doublet at 9.55 ppm as the aldehyde resonance of acrolein, the multiplet at 6.35 ppm as the trans β hydrogen, doublet at 6.47 ppm as the cis β hydrogen, and doublet at 6.625 ppm as the α hydrogen resonance of acrolein; the quartet at 9.65 ppm as the aldehyde resonance of acetaldehyde; and last, the overlapping triplets at 6.20 and 6.17 ppm as the hydroxyl resonance of the primary formaldehyde hemiacetals corresponding to PG and GLY, respectively. Chemical peak identification by the addition of authentic standards was performed extensively and published in our previous work.26 These spectra were obtained using EC2 at 11 W.
The aerosol levels of 1a–3 were quantified by 1H NMR, using the internal standard 2,3,5,6-tetrachloro-4-nitrobenzene. Concentrations were normalized by dividing by the mass of e-liquid consumed (Supporting Information). The peaks corresponding to compounds 1a–b were integrated together because of their overlapping peaks. In EC1 (sub-Ohm), compounds 1a–b were the only detectable target products by 1H NMR from aerosolized PG/GLY (Figure 3). However, the addition of 10% TA afforded 80–162% increases in 1a–b, as well as a detectable level of 2 in the aerosol. The relatively large error bars observed for the EC1 results are due to the fact that the relatively low levels of aldehydes produced were close to the limit of detection of the NMR technique. Although sub-Ohm devices typically produce lower concentrations of aerosol aldehyde products, they typically deliver much greater relative levels of PG and GLY to the user.28 The EC2 device thus produced orders of magnitude greater levels of 1a–3 (at 11 W) as compared to EC1 (no TA added). The inclusion of 10% TA in the EC2 e-liquid led to product increases of 185, 149, and 173%. Using EC2 at 9 W, aerosolized PG/GLY alone afforded no detectable levels of 1a–3. However, the addition of 10% TA afforded 1a–b, 2, and 3 in measurable amounts of 0.09, 0.004, and 0.003 mg/g, respectively. Thus, in the case of each e-cigarette, the e-liquids containing 10% TA exhibited a clear trend of enhanced relative levels of aldehydes compared to those containing only PG/GLY.
Figure 3.
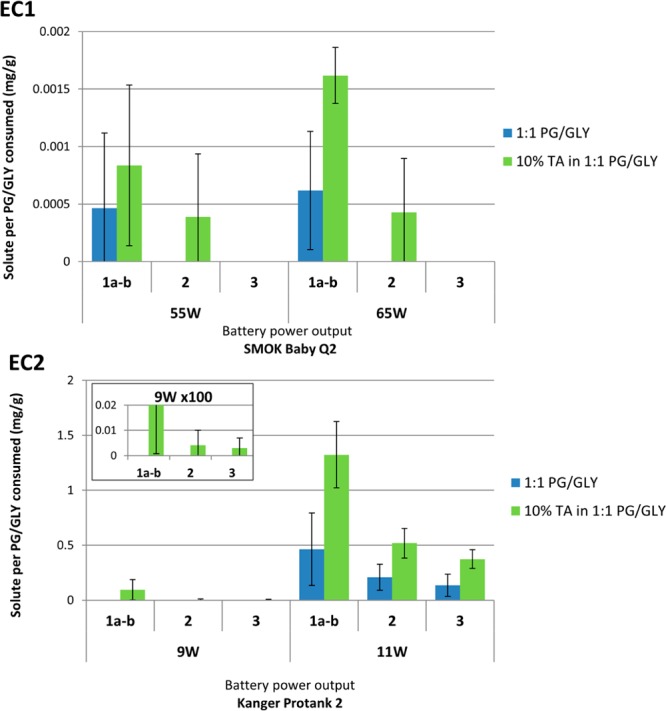
Concentrations of compounds 1a–3 in the aerosolization of PG/GLY and increased levels with the addition of 10% TA. The blue bar represents the amount of product (mg solute/g solution consumed) formed from aerosolized PG/GLY e-liquid. The green bar represents the amount of products formed from aerosolized TA/PG/GLY e-liquid. The inset displays the results from EC2 at 9 W, expanded by 100 times. 1a, 1b, 2, and 3 represent PG formaldehyde hemiacetal, GLY formaldehyde hemiacetal, acrolein, and acetaldehyde, respectively. Errors bars were calculated by one standard deviation. The enhanced concentration of 1a–3 was significant under all conditions except in the case of 1a–b generated by EC1 at 55 W (see the Supporting Information).
Origin of the Enhanced Product Formation
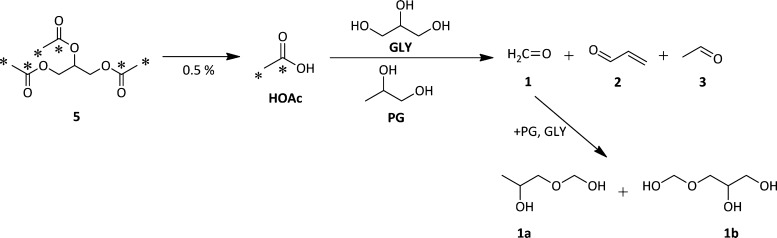
In order to best inform manufactures, regulatory agencies, and users of potential health risks, it is imperative to determine the sources of increased levels of 1–3. Aerosols derived from PG/GLY containing either 10% 13C3-TA (4) or 10% 13C6-TA (5) e-liquids were analyzed by 1H NMR and 13C NMR. The 13C NMR of the 10% 13C6-TA (5, acetate-labeled) aerosol displays a prominent peak at 172 ppm corresponding to the carbonyl peak of acetic acid. Importantly, this was the only 13C-labeled product observed, and it was not detectable in the 10% 13C3-TA-derived aerosol (4) spectrum. This indicates that ester hydrolysis of TA occurs to form HOAc during aerosolization. The formation of HOAc has the lowest energy barrier of the initial steps in the pyrolysis pathways of TA.23,29
Importantly, the degradation of PG and GLY is well-known to be catalyzed by acid and can lead to increased levels of 1–3.30,31 Therefore, we can conclude that TA promotes aldehyde formation in e-cigarettes by producing HOAc that serves as a catalyst to enhance PG and GLY reactivity (Scheme 2). This was confirmed by analyzing the aerosol derived from a control e-liquid consisting of a 1:1 PG/GLY solution containing 0.5% HOAc, the level of HOAc produced in the experiments using the acetate-labeled TA, 5. Figure 4 reveals that the 1a–3 aerosol spectrum derived from the HOAc/PG/GLY e-liquid exhibits enhanced 1a–3 levels, as is consistent with the findings from the TA/PG/GLY e-liquid (Figure 2).
Scheme 2. TA in E-Cigarettes Leads to HOAc Formation and Subsequent Protonation of PG/GLY To Catalyze the Formation of Products Such as 1–3.
This was confirmed via the use of 13C-labeled TA as the predominant pathway observed under the conditions used herein.
Figure 4.
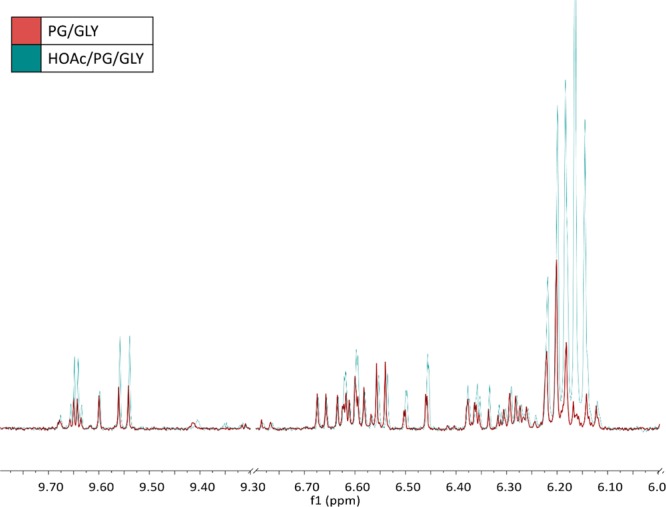
Overlay of 1H NMR spectra of aerosolized (red) PG/GLY e-liquid and (blue) 0.5% HOAc/PG/GLY e-liquid. The triplet at 6.20 ppm was identified as 1a. The triplet at 6.17 was identified as 1b. The doublet at 9.55 ppm, multiplet at 6.35 ppm, doublet at 6.47 ppm, and doublet at 6.625 ppm were identified as 2. The quartet at 9.65 ppm was identified as 3. In the presence of HOAc there is a visible increase in peaks corresponding to 1a–3. These spectra were obtained using EC2 at 11 W.
Finally, we found that the presence of HOAc leads to greater production of the GLY-derived formaldehyde hemiacetal 1b as compared to the PG formaldehyde hemiacetal 1a (Figure 4). Protonation of GLY has been reported to significantly lower the activation energy of its dehydration from 65–71 to 20–25 kcal mol–1. These results indicate that e-liquids containing TA and higher GLY/PG ratios may be relatively more prone to the enhanced production of formaldehyde and related products.
Conclusions
Herein, we have shown that the addition of TA to PG/GLY e-liquid affords higher levels of formaldehyde hemiacetals (1a–b), acrolein (2), and acetaldehyde (3) by releasing HOAc. This occurs via HOAc formation from TA, followed by acid catalysis of PG/GLY degradation. Although TA may be a direct source of aldehydes, we did not observe this under the conditions herein. One limitation of this study includes not quantifying gaseous formaldehyde because of the method of collection and analysis. However, our previous research has shown that an increase in 1a–b concentration is proportional to an increase in gaseous formaldehyde (1) production.32 Further related investigations involving additional e-liquid formulations are currently under study in our labs.
Methods
Electronic Cigarette Devices
Two devices were used for aerosolization.
EC1
A SMOK Alien 220 W variable voltage/variable wattage/temperature control (VV/VW/TC) battery was fitted with a SMOK Baby containing a Q2 0.4 Ω single vertical coil.
EC2
A SMOK Alien 220W VV/VW/TC battery was fitted with a Kanger Protank 2 Clearomizer containing a MT32 2.2 Ω single horizontal coil.
Synthesis of 13C-Labeled TA
13C3-GLY (Sigma-Aldrich) was converted to C6-13C3H14O6 (13C3-TA, 4) by acetic anhydride and pyridine (25 °C, 22 h). Purification was performed by column chromatography, followed by solvent evaporation under reduced pressure to afford the liquid product. Purity was confirmed by 1H NMR and 13C NMR. GLY was converted to C3-13C6H14O6 (13C6-TA, 5) by 13C4 acetic anhydride (Cambridge Isotopes) and pyridine (25 °C, 22 h). Purification was performed by column chromatography, followed by solvent evaporation under reduced pressure to afford the liquid product. Purity was confirmed by 1H NMR and 13C NMR.
E-Liquid Preparation and Avoidance of Dry Coils and Burnt E-Liquid
Each device was filled with e-liquid to the highest level according to the manufacturers’ recommendation.
PG/GLY Solution
A 1:1 ratio (by volume, v/v %) of PG/GLY was mixed in-house from ACS-grade PG and GLY. EC1 and EC2 were filled with a mixture of 1.0 mL PG and 1.0 mL GLY, respectively.
10% TA Solution
A 1:1 ratio of PG/GLY (v/v %) was mixed in-house with an addition of 10% (v/v %) ACS-grade TA.
10% 13C3-TA (4) Solution
A 1:1 ratio of PG/GLY (v/v %) was mixed in-house with an addition of 10% (v/v %) synthesized 4.
10% 13C6-TA (5) Solution
A 1:1 ratio of PG/GLY (v/v %) was mixed in-house with an addition of 10% (v/v %) synthesized 5.
Throughout each vaping session, ample e-liquid was maintained to cover the wicking material. After each session, the e-liquid was replaced with a fresh solution. New coils were also used in each session. Each device was studied at two wattages that were within self-reported user preferences (Supporting Information) as well as within the range of the manufacturers’ recommendation.
Collecting the Aerosol
The e-cigarette aerosol consists of liquid particles suspended in the gas phase.33 The aerosol produced was passed through a dry cold trap submerged in a dry ice/acetone bath (−76 °C ± 2 °C), followed by an impinger of 0.6 mL DMSO-d6 connected to a CH Technologies single cigarette smoking machine (SCSM-STEP). Each vaping session consisted of 10 puffs. The SCSM-STEP was set to the CORESTA program, which has a square shape puff profile, a 3 s puff period, and a 55 mL puff volume. In this study, the puff interval was set to 1 min to aid cooling of the heating coils between puffs. EC1 was tested in triplicate at 55 and 65 W. EC2 was tested in triplicate at 9 and 11 W. The aerosolization of 13C3-TA (4) e-liquid and 13C6-TA (5) e-liquid was each performed once with EC1 at 65 W and once with EC2 at 11 W. After each puff, DMSO-d6 from the impinger was used to collect the aerosols that had condensed inside the cold trap. The dissolved aerosol (0.425 mL) was placed in a Wilmad 400 MHz NMR tube. An internal standard was added via a 40 μL aliquot of a 10.01 mM 2,3,5,6-tetrachloro-4-nitrobenzene solution in DMSO-d6.
Analysis by NMR
NMR spectra were obtained with a Bruker 400 MHz AVANCE II+ spectrometer.
1H NMR
1H NMR spectra were obtained using a 30° pulse angle, 10 s relaxation delay, and 256 acquisitions.
13C NMR
Using a 30° pulse angle, 2 s relaxation delay, and 2048 acquisitions, 13C NMR spectra were obtained for the sample of (i) 10% 13C3-TA (4) solution, (ii) 10% 13C6-TA (5) solution, and (iii) 10% TA solution for EC1 at 65 W and for EC2 at 11 W. Data were processed and analyzed using the software, MNova.
Acknowledgments
We thank the NIH and the FDA for their support via award R01ES025257. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the FDA. We express sincere appreciation to Dr. Jorge Escobedo and Katherine Cook for generosity with their time and knowledge.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b00842.
Detailed synthesis procedures, 1H and 13C NMR spectral data, and sample collection data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Singh T.; Arrazola R. A.; Corey C. G.; Husten C. G.; Neff L. J.; Homa D. M.; King B. A. Tobacco Use Among Middle and High School Students - United States, 2011-2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 361–367. 10.15585/mmwr.mm6514a1. [DOI] [PubMed] [Google Scholar]
- Bold K. W.; Kong G.; Camenga D. R.; Simon P.; Cavallo D. A.; Morean M. E.; Krishnan-Sarin S. Trajectories of e-cigarette and conventional cigarette use among youth. Pediatrics 2018, 141, e20171832. 10.1542/peds.2017-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D.; Davis K. C.; Cox S.; Bradfield B.; King B. A.; Shafer P.; Caraballo R.; Bunnell R. Reasons for current E -cigarette use among U.S. adults. Prev. Med. 2016, 93, 14–20. 10.1016/j.ypmed.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare S.; Nemati M.; Zheng Y. A systematic review of consumer preference for e-cigarette attributes: Flavor, nicotine strength, and type. PLoS One 2018, 13, e0194145. 10.1371/journal.pone.0194145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bold K. W.; Kong G.; Cavallo D. A.; Camenga D. R.; Krishnan-Sarin S. Reasons for Trying E-cigarettes and Risk of Continued Use. Pediatrics 2016, 138, e20160895. 10.1542/peds.2016-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinouani S.; Pereira E.; Tzourio C. Electronic Cigarette Use in Students and Its Relation with Tobacco-Smoking: A Cross-Sectional Analysis of the i-Share Study. Int. J. Environ. Res. Public Health 2017, 14, 1345. 10.3390/ijerph14111345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg C. J. Preferred flavors and reasons for e-cigarette use and discontinued use among never, current, and former smokers. Int. J. Public Health 2016, 61, 225–236. 10.1007/s00038-015-0764-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaporizers, e-cigarettes, and other electronic nicotine delivery systems (ENDS). https://www.fda.gov/TobaccoProducts/Labeling/ProductsIngredientsComponents/ucm456610.htm (accessed Jan 10, 2018).
- Info Electronic Cigarettes . The Safety Of E-Liquid, What We Know Now. https://info-electronic-cigarette.com/e-juice/e-liquid-safety (accessed April 12, 2018).
- Better Than Food-Grade in Everything You Need to Know About VaporFi E Juices! https://www.vaporfi.com/blog/everything-you-need-to-know-about-vaporfi-e-juices/ (accessed April 16, 2018).
- Khlystov A.; Samburova V. Flavoring Compounds Dominate Toxic Aldehyde Production during E-Cigarette Vaping. Environ. Sci. Technol. 2016, 50, 13080–13085. 10.1021/acs.est.6b05145. [DOI] [PubMed] [Google Scholar]
- Lerner C. A.; Sundar I. K.; Yao H.; Gerloff J.; Ossip D. J.; McIntosh S.; Robinson R.; Rahman I. Vapors Produced by Electronic Cigarettes and E-Juices with Flavorings Induce Toxicity, Oxidative Stress, and Inflammatory Response in Lung Epithelial Cells and in Mouse Lung. PLoS One 2015, 10, e0116732. 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthumalage T.; Prinz M.; Ansah K. O.; Gerloff J.; Sundar I. K.; Rahman I. Inflammatory and Oxidative Responses Induced by Exposure to Commonly Used e-Cigarette Flavoring Chemicals and Flavored e-Liquids without Nicotine. Front. Physiol. 2018, 8, 1130. 10.3389/fphys.2017.01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff J.; Sundar I. K.; Freter R.; Sekera E. R.; Friedman A. E.; Robinson R.; Pagano T.; Rahman I. Inflammatory Response and Barrier Dysfunction by Different e-Cigarette Flavoring Chemicals Identified by Gas Chromatography-Mass Spectrometry in e-Liquids and e-Vapors on Human Lung Epithelial Cells and Fibroblasts. Appl. In Vitro Toxicol. 2017, 3, 28–40. 10.1089/aivt.2016.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh N. J.; Lawton R. I.; Hershberger P. A.; Goniewicz M. L. Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS). Tobac. Contr. 2016, 25, ii81–ii87. 10.1136/tobaccocontrol-2016-053205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K. E.; Voudris V. Do flavouring compounds contribute to aldehyde emissions in e-cigarettes?. Food Chem. Toxicol. 2018, 115, 212–217. 10.1016/j.fct.2018.02.059. [DOI] [PubMed] [Google Scholar]
- Eddingsaas N.; Pagano T.; Cummings C.; Rahman I.; Robinson R.; Hensel E. Qualitative Analysis of E-Liquid Emissions as a Function of Flavor Additives Using Two Aerosol Capture Methods. Int. J. Environ. Res. Public Health 2018, 15, 323. 10.3390/ijerph15020323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soussy S.; EL-Hellani A.; Baalbaki R.; Salman R.; Shihadeh A.; Saliba N. A. Detection of 5-hydroxymethylfurfural and furfural in the aerosol of electronic cigarettes. Tobac. Contr. 2016, 25, ii88–ii93. 10.1136/tobaccocontrol-2016-053220. [DOI] [PubMed] [Google Scholar]
- Farley S. M.; Schroth K. R. J.; Grimshaw V.; Luo W.; DeGagne J. L.; Tierney P. A.; Kim K.; Pankow J. F. Flavour chemicals in a sample of non-cigarette tobacco products without explicit flavour names sold in New York City in 2015. Tobac. Contr. 2018, 27, 170–176. 10.1136/tobaccocontrol-2016-053552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E-cig Express . Comments on MTS Vape Wizard by Flavour Art. http://www.ecigexpress.com/diy-e-liquid/diy-e-liquid-flavors/flavourart-usa/flavourart-tobacco/mts-vape-wizard-by-flavourart.html (accessed April 12, 2018).
- Perfumers Apprentice . Flavors—Non PG. https://shop.perfumersapprentice.com/c-133-flavors-non-pg.aspx (accessed April 12, 2018).
- Vreeke S.; Korzun T.; Luo W.; Jensen R. P.; Peyton D. H.; Strongin R. M. Dihydroxyacetone levels in electronic cigarettes: Wick temperature and toxin formation. Aerosol Sci. Technol. 2018, 52, 370–376. 10.1080/02786826.2018.1424316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laino T.; Tuma C.; Moor P.; Martin E.; Stolz S.; Curioni A. Mechanisms of Propylene Glycol and Triacetin Pyrolysis. J. Phys. Chem. A 2012, 116, 4602–4609. 10.1021/jp300997d. [DOI] [PubMed] [Google Scholar]
- Formaldehyde, 2-Butoxyethanol and 1-tert-Butoxy-2-propanol. IARC Monogr. Eval. Carcinog. Risks Hum. 2006, 88, 39–325. [PMC free article] [PubMed] [Google Scholar]
- Faroon O.; Roney N.; Taylor J.; Ashizawa A.; Lumpkin M. H.; Plewak D. J. Acrolein health effects. Toxicol. Ind. Health 2008, 24, 447. 10.1177/0748233708094188. [DOI] [PubMed] [Google Scholar]
- Jensen R. P.; Strongin R. M.; Peyton D. H. Solvent Chemistry in the Electronic Cigarette Reaction Vessel. Sci. Rep. 2017, 7, 42549. 10.1038/srep42549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. P.; Luo W.; Pankow J. F.; Strongin R. M.; Peyton D. H. Hidden Formaldehyde in E-Cigarette Aerosols. N. Engl. J. Med. 2015, 372, 392–394. 10.1056/nejmc1413069. [DOI] [PubMed] [Google Scholar]
- Korzun T.; Lazurko M.; Munhenzva I.; Barsanti K. C.; Huang Y.; Jensen R. P.; Escobedo J. O.; Luo W.; Peyton D. H.; Strongin R. M. E-Cigarette Airflow Rate Modulates Toxicant Profiles and Can Lead to Concerning Levels of Solvent Consumption. ACS Omega 2018, 3, 30–36. 10.1021/acsomega.7b01521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma C.; Laino T.; Curioni A.; Jochnowitz E.; Stolz S.. Reaction Pathways for the Pyrolysis of Glycerol, Propylene Glycol and Triacetin in the Gas Phase and at Solid Surfaces. Car-Parrinello Molecular Dynamics 2011, Barcelona, Spain, 2011.
- Clifton J. R.; Rossiter W. J. Jr; Brown P. W. Degraded aqueous glycol solutions: pH Values and the Effects of Common Ions on Suppressing pH Decreases. Sol. Energy Mater. 1985, 12, 77–86. 10.1016/0165-1633(85)90026-7. [DOI] [Google Scholar]
- Nimlos M. R.; Blanksby S. J.; Qian X.; Himmel M. E.; Johnson D. K. Mechanisms of Glycerol Dehydration. J. Phys. Chem. A 2006, 110, 6145–6156. 10.1021/jp060597q. [DOI] [PubMed] [Google Scholar]
- Salamanca J. C.; Munhenzva I.; Escobedo J. O.; Jensen R. P.; Shaw A.; Campbell R.; Luo W.; Peyton D. H.; Strongin R. M. Formaldehyde Hemiacetal Sampling, Recovery, and Quantification from Electronic Cigarette Aerosols. Sci. Rep. 2017, 7, 11044. 10.1038/s41598-017-11499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankow J. F. Calculating Compound Dependent Gas-Droplet Distributions in Aerosols of Propylene Glycol and Glycerol from Electronic Cigarettes. J. Aerosol Sci. 2017, 107, 9–13. 10.1016/j.jaerosci.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.