FIG 5.
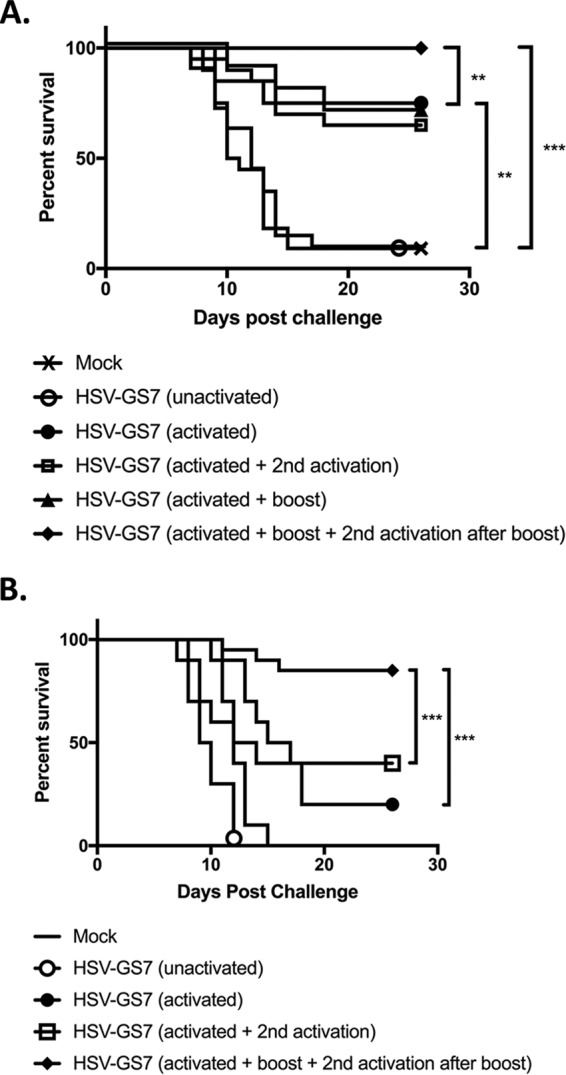
Effects of a second immunization or a second activation on HSV-GS7 efficacy. (A) Mice were inoculated on both rear feet with 50,000 PFU/mouse of HSV-GS7 or saline (mock; n = 10). HSV-GS7 groups were untreated (unactivated; n = 20); activated on day 1 (3 h after virus inoculation) by heat treatment at 45°C for 10 min in the presence of ulipristal (50 μg/kg administered i.p. at the time of inoculation) (activated; n = 20); activated on day 1 and reactivated 2 days later (activated + 2nd activation; n = 20); activated on day 1 and readministered 50,000 PFU/mouse of HSV-GS7 21 days later (activated + boost; n = 10); or activated on day 1, readministered 50,000 PFU/mouse of HSV-GS7 21 days after the first inoculation, and reactivated 3 h later (activated + boost + 2nd activation; n = 10). Twenty-one days after the last treatment, all the mice were challenged with a 20-fold lethal dose of wild-type HSV-1 strain 17syn+ applied to both rear feet. **, P ≤ 0.05; ***, P < 0.01. (B) Similar to panel A, except both initial and second immunizations were 5,000 PFU/mouse of HSV-GS7 (n = 10; for the second immunization, n = 20) (***, P ≤ 0.05). The data are presented as percent survival for each treatment group. See Table 1 and Fig. 6 for information on comparable experiments.
