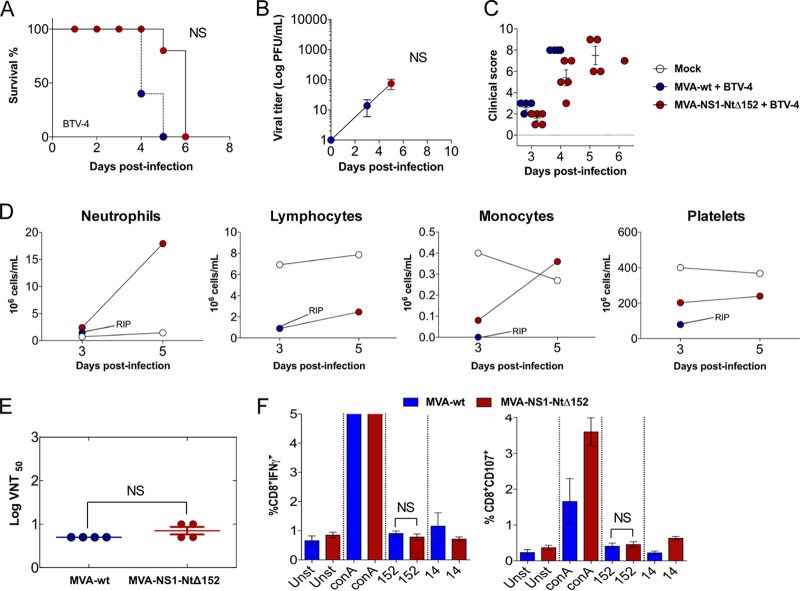
FIG 10.
MVA-NS1-NtΔ152 loses its protection capacity against BTV-4 in IFNAR−/− mice. (A) Survival rate after infection. Animals were inoculated with 107 PFU of MVA-NS1-NtΔ152 or MVA-wt as a negative control following a prime-boost strategy and challenged with a lethal dose of BTV-4. (B) Viral titers of BTV-4 recovered in blood of MVA-wt- (control) and MVA-NS1-NtΔ152-immunized IFNAR−/− mice after challenge. Each point represents the mean among the individual values of the viral titer of each animal, and SDs are shown as bars. (C) Postchallenge sickness score in control and MVA-NS1-NtΔ152-immunized IFNAR−/− mice challenged with BTV-4. (D) Blood parameters in control and MVA-NS1-NtΔ152-immunized IFNAR−/− mice infected with BTV-4. An autohematology analyzer (BC-5300 Vet; Mindray, China) was used in these experiments. Neutrophils, lymphocytes, monocytes, and platelets were analyzed at days 3 and 5 postinfection in pooled blood. Blood of noninfected and nonimmunized animals (mock) was used for reference values. (E) BTV-4 neutralizing antibody detection in control and MVA-NS1-NtΔ152-immunized mice by VNT. Neutralization titers in sera of immunized and control animals 10 days postboost are shown. SDs are shown as error bars. Asterisks indicate statistical significance calculated using the nonparametric Mann-Whitney test (P < 0.05). (F) Intracellular staining of IFN-γ (left) or CD107a (right) in CD8 T cells of MVA-NS1-NtΔ152 immunized animals. 10 days after the second immunization, spleens were harvested and the splenocytes were stimulated with NS1-152 peptide, using concanavalin A as a nonspecific stimulus, NS1-14 peptide as an irrelevant peptide, and RPMI 1640 as a negative control (unstimulated). At 24 h poststimulation, intracellular IFN-γ production was analyzed in CD8 T cells and at 6 h poststimulation, the indirect marker of cytotoxicity CD107a was also measured in CD8 T cells by flow cytometry. The results represent the averages from 4 mice ± SDs.