Figure 1.
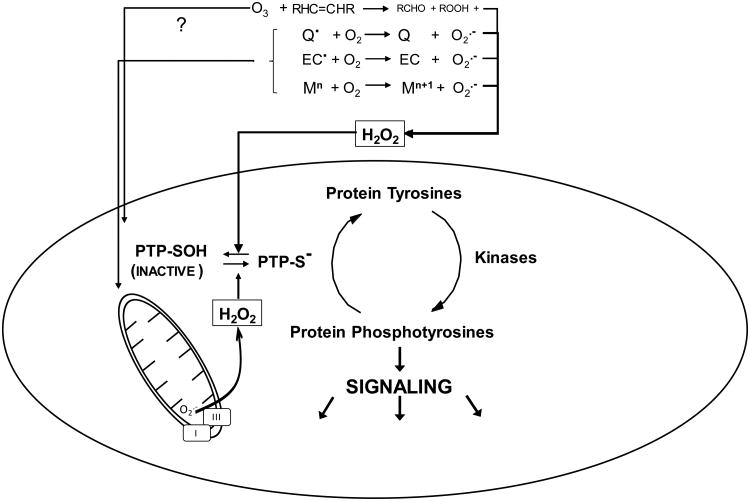
All Roads Lead to H2O2: A broad range of physichochemically disparate environmental agents induce elevations in intracellular H2O2 through multiple mechanisms. The ubiquitous photochemical air pollutant ozone (O3) is a potent oxidizer that reacts readily with alkenyl groups in membrane fatty acids to produce primarily aldehydes and H2O2, as well as lesser quantities of reducible products such as lipid hydroperoxides. Certain organic compounds such redox active quinones (Q) can undergo single electron reduction, catalyzed enzymatically or by reaction with extracellular or intracellular cofactors, to produce semiquinone free radicals that can donate an electron to diatomic oxygen to produce superoxide (O2.-). In addition, certain nanoscale particulate surfaces such as those of elemental carbon particles (EC) are known to have carbon-centered radicals that similarly reduce oxygen to form O2.-. Transition metals with two or more adjacent valence states (e.g., Fe, Cu, V, Ni) may be oxidized by O2 and form O2.- and be re-reduced in a cyclical manner by compounds such as ascorbate. Many environmental agents, including quinones and metal ions, impair mitochondrial respiration where mitochondrial complexes I and III are known sources of O2.-. O2.- is a short lived species, undergoing rapid spontaneous or enzymatically catalyzed dismutation to form H2O2. Certain regulatory proteins such as the protein tyrosine phosphatases (PTP) are subject to reversible redox modification by H2O2 wherein the thiolate anion (-S-) on the cysteine is oxidized to the sulfenic form (inactivating the PTP). Redox of direct electrophilic PTP inactivation leads to a loss of signaling quiescence.